The elements in the periodic table are broadly classified as metals, non-metals, and metalloids. Although metals and non-metals have defined properties, metalloids are classified as an intermediate of both metals and non-metals with shared physical and chemical properties.
Let’s see their properties and trends in the periodic table one by one.
1. Metals
About 80% of the elements in the periodic table are metals. These elements are present on the left side and the center of the periodic table.
The properties of metals are the elements that are malleable and ductile, possess luster, are good conductors of heat, and electricity, and have high densities.
Metals usually have high melting and boiling points and are generally solids at room temperature. But there are exceptions like Mercury is one of the metals which is liquid at room temperature.
Bromine is another metal that is present in the liquid state at room temperature. Apart from Bromine and Mercury, four other elements are also liquid at a temperature slightly warmer than room temperature. They are Francium, Gallium, Cesium, and Rubidium.
2. Non-Metals
Non-metals are much less in number than metals. There are over about 20 non-metals.
Non-metals are located at the top right hand side of the Periodic Table. The non-metallic character increases going from left to right and the metallic character increases going down a group.
Non-metals have low melting and boiling points. They are usually solids or gases at room temperature. Non-metals are neither malleable nor ductile. They are poor conductors of heat and electricity.
3. Metalloids
Metalloids are categorized as an intermediate and since there is a mix of physical as well as chemical properties, there is no sharp dividing line between metals and non-metals. As a result, metalloids are also called semi-metals.
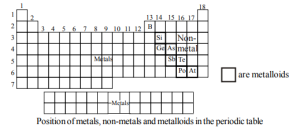
A zig-zag line separates metals from non-metals as shown in the figure. The borderline elements such as silicon, germanium, arsenic, antimony, and tellurium exhibit characteristic properties of metals as well as non-metals.
View this lecture to better understand the properties of metals, non-metals, and metalloids- https://youtu.be/lA6LOE2AhRQ.
Electropositivity and Metallic Character
The tendency of atoms of an element to lose electrons and form positive ions is known as electropositivity.
Also check electronegativity.
A more electropositive element has a more metallic character
Variation in a period, from left to right electropositivity decreases. This is due to an increase in ionization enthalpy (decrease in size) along a period which, makes the loss of electrons difficult.
For example, in the second period, Lithium and Beryllium are metals. Boron is a semimetal whereas Carbon, Nitrogen, Oxygen, and Fluorine are non-metals.
Variation in a group: On going from top to bottom electropositive nature increases hence metallic character increases on moving down the group.
In group 14 first two elements (Carbon and Silicon) are non-metals, the third element (Germanium) is a metalloid whereas the next elements (Tin and Lead) are metals.
INERT PAIR EFFECT
It is generally observed in groups 13, 14, and 15. According to this effect, as we descend down in a group, the two electrons of the s-orbital of the valence shell become inert, do not easily take part in bonding and the element shows its oxidation state two units less than the group oxidation number.
e.g. Al has stable oxidation state = +3
Ga has stable oxidation state = +3
In shows both = +1 and +3 oxidation state
Tl shows stable oxidation state = +1
Reason: On moving from top to bottom the effective nuclear charge increases due to which the penetration power of s-orbital and p-orbital also increases and hence s-orbital electrons are less available for bonding. This is called the inert pair effect of s-orbital electrons and therefore, on moving top to bottom lesser oxidation states are more stable.
‘Inert pair effect’ refers to the resistance of a pair of ‘s’ electrons to participate in covalent bond formation.
The inert pair effect among groups 14 and 15 elements is observed. Sn2+ and Pb2+; Sb3+ and Bi3+ are more stable in the actual oxidation state minus two due to the inert pair effect. The s-electrons remain paired with the oxidation state two lower than the characteristic oxidation state of the group.
d-block elements: Variable valency also occurs with elements in the d-block. This arises from using different numbers of d electrons for bonding, so in this case, the valency can change in steps of one. Read here for classification of elements into s, p, d, and f blocks.
![]()
To know more, visit: https://youtu.be/G7cZwMrLz20.

mexican rx online
https://cmqpharma.online/# reputable mexican pharmacies online
purple pharmacy mexico price list
pharmacies in mexico that ship to usa: mexico pharmacy – buying from online mexican pharmacy
Good info and straight to the point. I don’t know if this is truly the best place to ask
but do you folks have any thoughts on where to get some professional writers?
Thx 🙂 Escape room
You have noted very interesting details! ps
nice website..
buying prescription drugs in mexico mexican pharmaceuticals online buying prescription drugs in mexico online
mexico pharmacies prescription drugs: purple pharmacy mexico price list – best online pharmacies in mexico
https://indiapharmast.com/# indianpharmacy com
mexican border pharmacies shipping to usa mexico pharmacy buying prescription drugs in mexico online
mexico pharmacies prescription drugs: mexican mail order pharmacies – mexican pharmacy
mexican drugstore online mexican pharmaceuticals online mexican mail order pharmacies
Online medicine home delivery: buy medicines online in india – india pharmacy
http://foruspharma.com/# mexican online pharmacies prescription drugs
safe canadian pharmacy: certified canadian pharmacy – onlinecanadianpharmacy
pharmacy in canada: my canadian pharmacy – canadian drugs pharmacy
northern pharmacy canada safe canadian pharmacy canadian pharmacy antibiotics
mexican border pharmacies shipping to usa: mexico pharmacy – mexico drug stores pharmacies
indianpharmacy com: india online pharmacy – indian pharmacy
An impressive share! I have just forwarded this onto a coworker who was conducting a little homework on this. And he actually ordered me dinner simply because I found it for him… lol. So allow me to reword this…. Thanks for the meal!! But yeah, thanx for spending some time to discuss this matter here on your web page.
https://foruspharma.com/# mexico drug stores pharmacies
medication from mexico pharmacy: mexican online pharmacies prescription drugs – buying prescription drugs in mexico
Good write-up. I definitely love this site. Stick with it!
http://paxloviddelivery.pro/# paxlovid for sale
Greetings! Very useful advice in this particular post! It’s the little changes that produce the largest changes. Thanks for sharing!
https://amoxildelivery.pro/# where to buy amoxicillin 500mg
I would like to thank you for the efforts you have put in penning this site. I’m hoping to check out the same high-grade content from you later on as well. In truth, your creative writing abilities has encouraged me to get my very own site now 😉
https://doxycyclinedelivery.pro/# generic doxycycline online
A fascinating discussion is worth comment. There’s no doubt that that you need to write more on this subject matter, it may not be a taboo matter but usually people do not speak about such issues. To the next! Best wishes.
https://doxycyclinedelivery.pro/# doxycycline 20 mg coupon
I quite like reading a post that can make people think. Also, many thanks for allowing me to comment.
http://ciprodelivery.pro/# buy cipro online without prescription
Nice post. I learn something totally new and challenging on sites I stumbleupon everyday. It will always be interesting to read articles from other authors and use something from other web sites.
When I initially commented I appear to have clicked the -Notify me when new comments are added- checkbox and from now on whenever a comment is added I recieve four emails with the same comment. Perhaps there is a way you can remove me from that service? Kudos.
http://clomiddelivery.pro/# can i buy clomid online
http://amoxildelivery.pro/# amoxicillin 500 mg tablet price
This is a topic that’s close to my heart… Thank you! Where can I find the contact details for questions?
This excellent website certainly has all of the information I wanted about this subject and didn’t know who to ask.
https://ciprodelivery.pro/# п»їcipro generic
http://clomiddelivery.pro/# how to buy clomid without dr prescription
This site was… how do you say it? Relevant!! Finally I have found something which helped me. Thanks a lot.
I’m very happy to uncover this great site. I wanted to thank you for ones time just for this wonderful read!! I definitely appreciated every little bit of it and I have you book marked to check out new information in your site.
Oh my goodness! Incredible article dude! Thank you, However I am having difficulties with your RSS. I don’t understand the reason why I cannot subscribe to it. Is there anyone else having similar RSS problems? Anyone that knows the solution can you kindly respond? Thanks.
An outstanding share! I have just forwarded this onto a co-worker who was conducting a little homework on this. And he in fact bought me dinner simply because I found it for him… lol. So let me reword this…. Thank YOU for the meal!! But yeah, thanks for spending time to discuss this subject here on your internet site.