What is Atomic Radius?
Atomic Radius (or Atomic Radii) is defined as the distance from the center of the nucleus to the outermost shell containing electrons and is measured in Nanometers (10-9m).
However, it is almost impossible to determine the exact radius of an atom because of the following reasons:
- Electron cloud has no sharp boundaries.
- It is not possible to isolate a single atom for the determination of radius.
- If a group of atoms is considered, the probability distribution of the electron may be influenced by the neighboring atoms.
Types of Atomic Radii
Depending on the classification of the element as a metal or non-metal, three different types of atomic radii are used. They are:
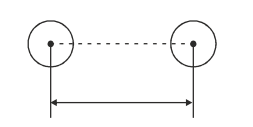
1. Covalent Radii:
It is defined as half the distance between the nuclei of two covalently bonded atoms.

- As the percentage s-character increases in any bond, the atomic radius (covalent radius) decreases. The atomic radius of C (sp3) > C (sp2) > C (sp) hybridized atom in a molecule.
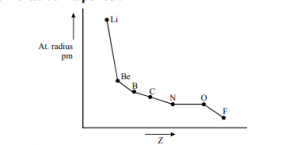
- Variation in a group: Atomic radius increases on descending a group in the periodic table because of the addition of a new shell at each step.
- Variation in a period: On moving across a period from left to right in a periodic table because the magnitude of nuclear charge increases, while the addition of electrons takes place in the same shell.
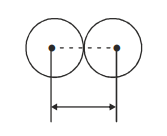
2. Metallic Radii or Crystal Radius:
It is defined as one-half of the distance between the nuclei of two adjacent atoms in a metallic lattice. Metallic radius is independent of the type of packing.
3. Vanderwaal Radius:
It is defined as the internuclear separation when the valence shell of two atoms is in nonbonding contact.
Variation in atomic radius in a period:
Comparison of Atomic Radii:
Vanderwaal Radius > Metallic Radius > Covalent Radius
To know more about atomic radius and its types follow- https://youtu.be/lA6LOE2AhRQ
Ionic Radii
Ionic Radii is defined as the distance from the center of the nucleus upto where it exerts its influence on its electron cloud.
NOTE: The radius of an anion is always larger than its parent atom and the radius of a cation is always smaller than its parent atom.
![]()
Comparison of ionic radius in isoelectronic species
Isoelectronic species have the same number of electrons.
Example: Al3+, Mg2+, Na+, O2–, and F– are isoelectronic species because they all have 10 electrons.
As the magnitude of the positive charge increases, the ionic radius decreases and the ionic radius increases with an increase in the magnitude of the negative charge.
Order of ionic radii: Al3+ < Mg2+ < Na+ < F– < O2– < N3– < Ne
(Noble gases have the highest radii among their isoelectronic species because Vanderwaal’s radius is measured for noble gases)
What is Ionization Enthalpy?
Ionization enthalpy is defined as the minimum amount of energy required to remove the most loosely bounded electron from an isolated gaseous atom. It is expressed in units of energy: eV/atom, Kcal/mol, kJ/mol.
1 eV/atom = 1.602 × 10-16 kJ/ atom = 1.602×10-16 × 6.02×1023 kJ/ mol = 96.4 ×106 kJmol-1
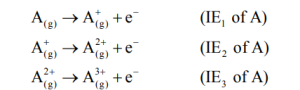
- Ionization is always an endothermic process i.e. ΔH is positive.
- Successive ionization energies always increase.
IE1 < IE2 < IE3 ……..
This is because, after the removal of an electron, the attraction of the nucleus on remaining electrons increases i.e. effective nuclear charge increases, and hence the ionization energy also increases.
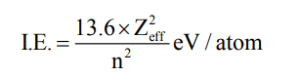
Factors governing ionization enthalpy
- Atomic size/radius: As the atomic size increases, the ionization energy decreases. This is because with increase in size the outermost e– lies farther from the nucleus and it requires a lesser amount of energy to remove the e– lying at a larger distance from the nucleus.
- Effective nuclear charge: As the effective nuclear charge increases, the ionization energy increase. This is due to the increased attraction of the nucleus towards the electrons.
- Penetration effect of electrons: As the penetration effect increases, ionization energy also increases. The penetration effect follows the order : s > p > d > f. So the e– in s-subshell would lie closer to the nucleus and much firmly held at the nucleus.
Example: IE1 of Mg > IE1 of Al. Because in the case of Mg, the electron is to be removed from the 3s subshell, and in Al, the electron is to be removed from the 3p subshell. - Screening effect of inner shell electrons: As the screening effect increases, ionization enthalpy decreases because due to an increase in shielding of the nuclear charge by the inner electrons, a lesser amount of energy is required to remove an electron from the valence shell.
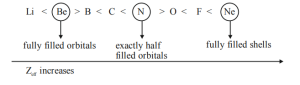
- Electronic configuration of the element: If an atom has exactly half-filled or completely filled orbitals, the atoms with such configuration are more stable and require more energy to remove the electron. Hence, ionization energy increases with the increase in stability of electronic configuration.
Example: N(1s22s22p3) and O(1s22s22p4).
Nitrogen has exactly half-filled electronic configuration hence more stable than oxygen and has higher ionization energy.
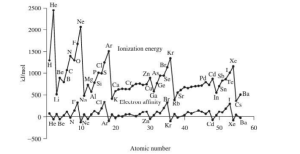
Variation of Ionization Energy in a period
On going from left to right in the periodic table, effective nuclear charge increases. Hence ionization enthalpy increases with increasing atomic number.
Variation of Ionization Energy in a group
Ionization energy decreases on descending down the group because of an increase in atomic size and decreased effective nuclear charge.
Key Takeaways:
- Maxima and minima occur for noble gases and alkali metals respectively.
- Elements with exactly half-filled configurations and fully filled shells have higher ionization energy than their immediate neighbors.
- More electropositive species have lesser ionization energies.
Electron Affinity (EA) and Electron Gain Enthalpy(ΔegH)
Electron Gain Enthalpy is defined as the amount of energy released or absorbed when an electron is added to an isolated gaseous atom.
![]()
whereas,
Electron Affinity is defined as the tendency of an atom to accept an incoming additional electron.
The relation between electron gain enthalpy and electron affinity is—
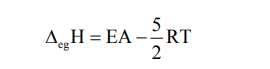
- First electron gain enthalpy is generally negative means addition of the first electron is an exothermic reaction.
- Second electron gain enthalpy is always positive for every element because adding an electron to an already negatively charged specie requires energy due to electron-electron repulsion between the anion and incoming electron. Therefore ΔH value will be positive.
Example:
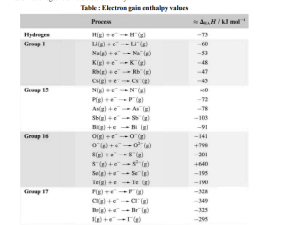
Key takeaways:
- Nitrogen has very low electron affinity because incoming electron experiences high electron – electron repulsion.
- A negative enthalpy but positive electron affinity corresponds to an exothermic process.
Variation in Electron Affinity
- Atomic size: As the size of the atom increases, the distance between the nucleus and outer e– increases therefore the forces of attraction between the nucleus and incoming e– decrease.
- Nuclear charge: With the increase in effective nuclear charge, the attraction between incoming e– and nucleus increases thereby increasing electron affinity.
- Electronic configuration: The elements having exactly half filled, fully filled or noble gas configuration have negative or zero electron affinity.

I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.