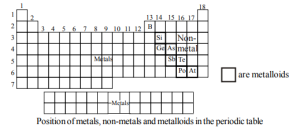
The elements in the periodic table are broadly classified as metals, non-metals, and metalloids. Although metals and non-metals have defined properties, metalloids are classified as an intermediate of both metals and non-metals with shared physical and chemical properties.
Let’s see their properties and trends in the periodic table one by one.
1. Metals
About 80% of the elements in the periodic table are metals. These elements are present on the left side and the center of the periodic table.
The properties of metals are the elements that are malleable and ductile, possess luster, are good conductors of heat, and electricity, and have high densities.
Metals usually have high melting and boiling points and are generally solids at room temperature. But there are exceptions like Mercury is one of the metals which is liquid at room temperature.
Bromine is another metal that is present in the liquid state at room temperature. Apart from Bromine and Mercury, four other elements are also liquid at a temperature slightly warmer than room temperature. They are Francium, Gallium, Cesium, and Rubidium.
2. Non-Metals
Non-metals are much less in number than metals. There are over about 20 non-metals.
Non-metals are located at the top right hand side of the Periodic Table. The non-metallic character increases going from left to right and the metallic character increases going down a group.
Non-metals have low melting and boiling points. They are usually solids or gases at room temperature. Non-metals are neither malleable nor ductile. They are poor conductors of heat and electricity.
3. Metalloids
Metalloids are categorized as an intermediate and since there is a mix of physical as well as chemical properties, there is no sharp dividing line between metals and non-metals. As a result, metalloids are also called semi-metals.
A zig-zag line separates metals from non-metals as shown in the figure. The borderline elements such as silicon, germanium, arsenic, antimony, and tellurium exhibit characteristic properties of metals as well as non-metals.
View this lecture to better understand the properties of metals, non-metals, and metalloids- https://youtu.be/lA6LOE2AhRQ.
Electropositivity and Metallic Character
The tendency of atoms of an element to lose electrons and form positive ions is known as electropositivity.
Also check electronegativity.
A more electropositive element has a more metallic character
Variation in a period, from left to right electropositivity decreases. This is due to an increase in ionization enthalpy (decrease in size) along a period which, makes the loss of electrons difficult.
For example, in the second period, Lithium and Beryllium are metals. Boron is a semimetal whereas Carbon, Nitrogen, Oxygen, and Fluorine are non-metals.
Variation in a group: On going from top to bottom electropositive nature increases hence metallic character increases on moving down the group.
In group 14 first two elements (Carbon and Silicon) are non-metals, the third element (Germanium) is a metalloid whereas the next elements (Tin and Lead) are metals.
INERT PAIR EFFECT
It is generally observed in groups 13, 14, and 15. According to this effect, as we descend down in a group, the two electrons of the s-orbital of the valence shell become inert, do not easily take part in bonding and the element shows its oxidation state two units less than the group oxidation number.
e.g. Al has stable oxidation state = +3
Ga has stable oxidation state = +3
In shows both = +1 and +3 oxidation state
Tl shows stable oxidation state = +1
Reason: On moving from top to bottom the effective nuclear charge increases due to which the penetration power of s-orbital and p-orbital also increases and hence s-orbital electrons are less available for bonding. This is called the inert pair effect of s-orbital electrons and therefore, on moving top to bottom lesser oxidation states are more stable.
‘Inert pair effect’ refers to the resistance of a pair of ‘s’ electrons to participate in covalent bond formation.
The inert pair effect among groups 14 and 15 elements is observed. Sn2+ and Pb2+; Sb3+ and Bi3+ are more stable in the actual oxidation state minus two due to the inert pair effect. The s-electrons remain paired with the oxidation state two lower than the characteristic oxidation state of the group.
d-block elements: Variable valency also occurs with elements in the d-block. This arises from using different numbers of d electrons for bonding, so in this case, the valency can change in steps of one. Read here for classification of elements into s, p, d, and f blocks.
![]()
To know more, visit: https://youtu.be/G7cZwMrLz20.

Introducing to you the most prestigious online entertainment address today. Visit now to experience now!