What are Quantum Numbers?
On solving the Schrodinger equation, ![]() the solutions obtained in form of the wave function are uniquely defined by a set of integers called quantum number n, l, and m.
the solutions obtained in form of the wave function are uniquely defined by a set of integers called quantum number n, l, and m.
Each quantum number gives information about specific things.
1. Principal Quantum Numbers (n)
It represents the energy of the bounded electron. Also, the number of shells and size of orbitals are indicated by this quantum number. Possible values of n are 1, 2, 3, ……
The higher the value of n, the higher the energy of the orbital, and the larger the size of the orbital. Radius and energy can also be predicted by the value of the principal quantum number. The total number of electrons in a shell = 2n2.
2. Azimuthal Quantum Number (l)
It measures the orbital angular momentum of the electron, particularly ‘ℓ’ describes sub-shells. It determines the shape of orbitals in a sub-shell.
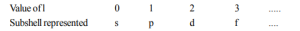
The possible value of ℓ is from 0 to (n – 1), where n is the principal quantum number. The number of lobes increases with an increasing value of ℓ.
Number of subshells in a shell = n
Example: How many subshells are present in the third shell?
Solution: The third shell means n = 3
Number of subshell = 3 (3s, 3p and 3d)
3. Magnetic Quantum Number (m or mℓ )
In presence of the magnetic field, the electron in a given sub-energy level orient themselves in certain specific regions in space around the nucleus It describes the position of orbital in space. The number of equivalent ways that can be oriented in space is (2ℓ + 1). The value of m can vary from –ℓ to +ℓ.
Example: How many orbitals are present in d-subshell?
Solution: Number of orbitals (2ℓ + 1) = [2(2) + 1] = 5 m values = -2, –1, 0, +1, +2
There will be five d-orbitals represented as:
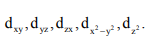
- Number of orbitals in nth shell = n2
- Maximum number of electrons in nth shell = 2n2
- Number of orbitals in a subshell = (2ℓ + 1)
- Maximum number of electrons in a subshell = 2 (2ℓ + 1)
4. Spin Quantum Number (s)
It describes the orientation of electron spin. The possible values of spin are +½ and –½. The spin of an electron may be of either orientation (clockwise or anticlockwise) with neither preferred.

I like this blog it’s a master piece! Glad I discovered this ohttps://69v.topn google.Raise your business