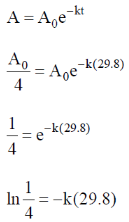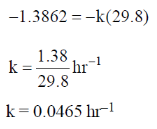IIT JAM Chemistry 2018
Previous Year Question Paper with Solution.
1. NaF, KF, MgO and CaO are crystalline solids. They have NaCl structure. Their lattice energies vary in the order
(a) NaF < KF < MgO < CaO
(b) KF < NaF < CaO < MgO
(c) MgO < CaO < NaF < KF
(d) CaO < MgO < KF < NaF
Ans. b
Sol. Lattice energy of any ionic compound is a function of charge on constituent cations, anions and internuclear distance between ions.
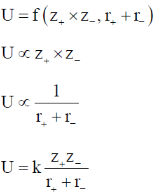
In MgO, Mg2+ and O2– ions have highest magnitude of charge product and smallest internuclear distance accounts for its largest lattice energy among other ionic compound
2. The number of crystal systems and the number of Bravais lattices are, respectively,
(a) 14 and 7
(b) 7 and 32
(c) 32 and 14
(d) 7 and 14
Ans. d
Sol. There are 7 crystal systems and 14 number of bravis lattices in solid state

3. 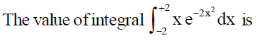
(a) 0
(b) 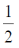
(c) 1
(d) 2
Ans. a
Sol. 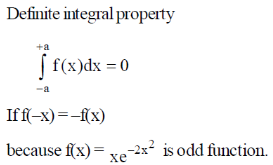
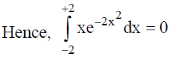
4. Carbonic anhydrase is an example of
(a) Hydrolysis enzyme
(b) Redox enzyme
(c) O2 transport protein
(d) Heme protein
Ans. a
Sol. Carbonic anhydrase is a enzyme that catalyse rapid interconversion of carbon dioxide and water to bicarbonate.
It is a hydrolysis enzyme.
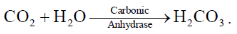
5. For adsorption of a gas on a solid suirface, the plot that represents Freundlich isotherm is (x = mass of gas, m = mass of adsorbent, P = pressure)
(a) 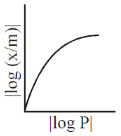
(b) 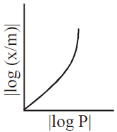
(c) 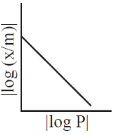
(d) 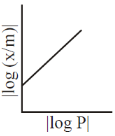
Ans. d
Sol. 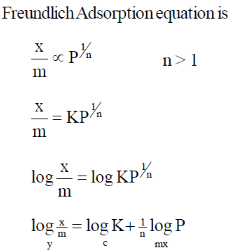
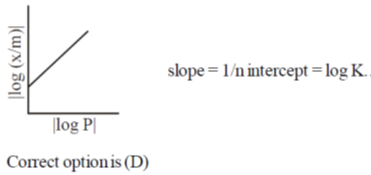
6. The compound that contains the most acidic hydrogen is
(a) H2C=CH2
(b) 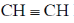
(c) 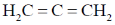
(d) H3C–CH3
Ans. b
Sol. Acidity of proton depends upon the hybrization of carbon to which it is attached. A sp hybridised carbon is very electronegative and can stablise easily negative charge on it
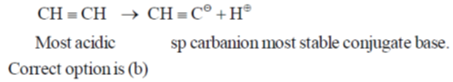
7. 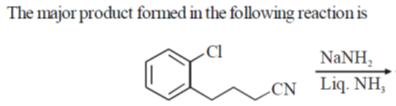
(a) 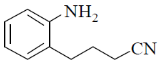
(b) 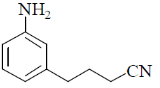
(c) 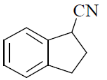
(d) 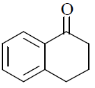
Ans. c
Sol. 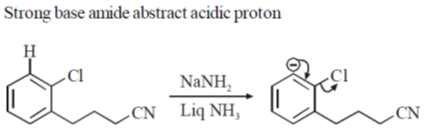
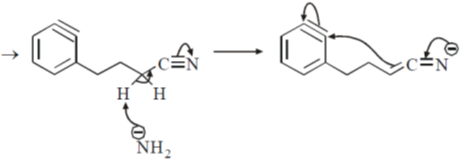
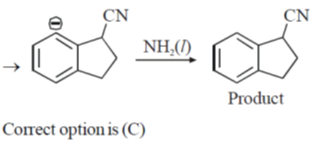
8. The CORRECT order of melting points of group 15 trifluorides is
(a) 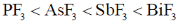
(b) 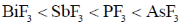
(c) 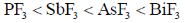
(d) 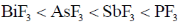
Ans. a
Sol. Melting point depends on strength of intermolecular forces of attractions between molecules.
As molecular mass increases, vander waal forces of attraction becomes higher and hence none energy is needed to convert into gaseous form from solid state therfore melting point increases
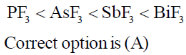
9. The C-2 epimer of D-glucose is
(a) D-Mannose
(b) D-Fructose
(c) D-Galactose
(d) D-Gulose
Ans. a
Sol. C–2 epimer mean configuration at C2 only is different and rest of the molecule is same.
C–2 epimer of D-glucose is D-Mannose.
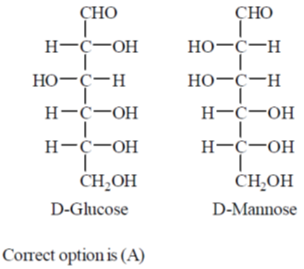
10. On hydrolysis, aluminium carbide produces
(a) CH4
(b) C2H6
(c) C2H4
(d) C2H2
Ans. a
Sol. Hydrolysis of aluminium carbide is given as
11. With respect to periodic properties, the CORRECT statement is
(a) Electron affinity order is F > O > Cl
(b) First ionisation energy order is Al > Mg > K
(c) Atomic radius order is N > P > As
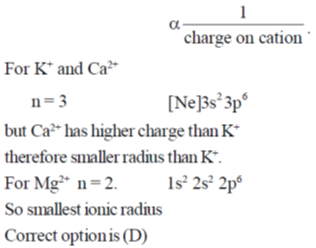
(d) Ionic radius order is K+ > Ca2+ > Mg2+
Ans. d
Sol. Ionic radius of any cation depends on its valance shell number, n and charge on cation
Ionic radius α principle quantum number, n
12. Among the following metal carbonyl species, the one with the highest metal-carbon back bonding is
(a) 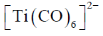
(b) 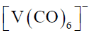
(c) 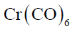
(d) 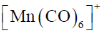
Ans. a
Sol. (A)Strength of Metal-Carbonyl back bonding depends on availability of electron density on metal.
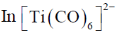 Ti is in –2 oxidation state and has highest donating tendency towards
Ti is in –2 oxidation state and has highest donating tendency towards  of carbonyl
of carbonyl
Therefore highest metal-carbon back bonding is observed with 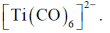
Correct option is (A)
13. With reference to the variation of molar conductivity 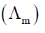 with concentration for a strong electrolyte in an aqueous solution, the CORRECT statement is
with concentration for a strong electrolyte in an aqueous solution, the CORRECT statement is
(a) The asymmetry effect contributes to decrease 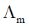 whereas the electrophoretic effect contributes to increase
whereas the electrophoretic effect contributes to increase 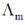
(b) The asymmetry effect contributes to increase 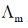 whereas the electrophoretic effect contributes to decrease
whereas the electrophoretic effect contributes to decrease 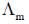
(c) Both asymmetry effect and electrophoretic effect contribute to decrease 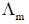
(d) Both asymmetry effect and electrophoretic effect contribute to increase 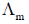
Ans. c
Sol. Correct option is (c)
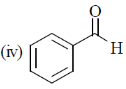
14. The CORRECT order of carbonyl stretching frequencies for the following compounds is
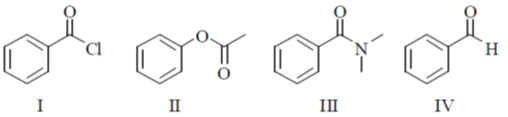
(a) II < I < III < IV
(b) I < III < II < IV
(c) IV < II < III < I
(d) III < IV < II < I
Ans. d
Sol. 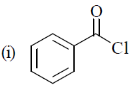 due to –I effect of Cl, carbonyl carbon becomes more deficient and hence
due to –I effect of Cl, carbonyl carbon becomes more deficient and hence 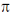 bond is strengthened so absorb highest wavenumber.
bond is strengthened so absorb highest wavenumber.
 lone pair of oxygen is in cross conjugation with CO group and phenyl sing and hence when lone pair donate to ring CO bond is strengthened (due to –I effect of =O+–) but when lone pair interact with CO,
lone pair of oxygen is in cross conjugation with CO group and phenyl sing and hence when lone pair donate to ring CO bond is strengthened (due to –I effect of =O+–) but when lone pair interact with CO,  bond becomes weak therefore it absorbs tower wavenumber than (I)
bond becomes weak therefore it absorbs tower wavenumber than (I)
 Ring
Ring  electron density interact with CO group and hence C=O bond becomes weak so absorb at lower wavenumber than (II)
electron density interact with CO group and hence C=O bond becomes weak so absorb at lower wavenumber than (II)
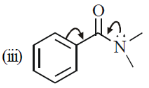 due to +R effect of phenyl and +M effect of N lone pair, C=O has double bond character only, weakest among other.
due to +R effect of phenyl and +M effect of N lone pair, C=O has double bond character only, weakest among other.
So absorb lowest wavenumber
III < IV < II < I
Correct option is (d)
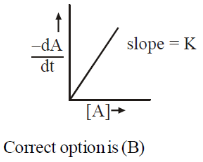
15. The reaction, A  Products, follows first-order kinetics. If [A] represents the concentration of reactant at time t, the INCORRECT variation is shown in
Products, follows first-order kinetics. If [A] represents the concentration of reactant at time t, the INCORRECT variation is shown in
(a) 
(b) 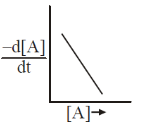
(c) 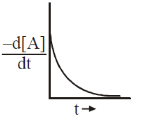
(d) 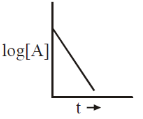
Ans. b
Sol. 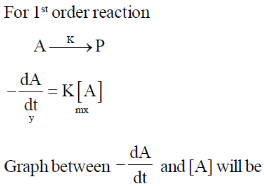
16. The behavior of Cl2 is closest to ideal gas behavior at
(a) 100 ºC and 10.0 atm
(b) 0 ºC and 0.50 atm
(c) 200 ºC and 0.50 atm
(d) –100 ºC and 10.0 atm
Ans. c
Sol. Any gas can behave like ideal gas at extremely high temperature and low pressure, therefore for Cl2 to behave ideally 200ºC and 0.50 atm is required.
Correct option is (c)
17. The decay modes of 14C and 14O are
(a) 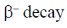
(b) positron emission
(c) 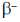 decay and positron emission, respectively
decay and positron emission, respectively
(d) positron emission and 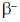 decay, respectively
decay, respectively
Ans. d
Sol. 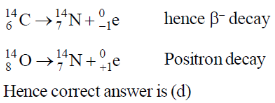
18. 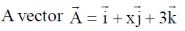 is rotated through an angle and is also doubled in magnitude resulting in
is rotated through an angle and is also doubled in magnitude resulting in 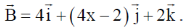 An acceptable value of x is
An acceptable value of x is
(a) 1
(b) 2
(c) 3
(d) 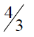
Ans. b
Sol. 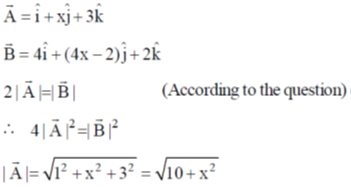
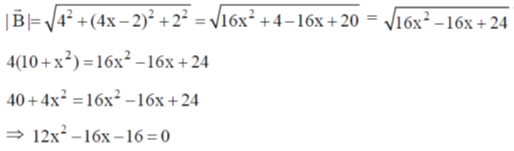
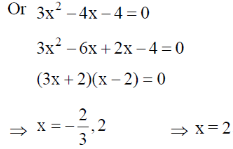
19. The CORRECT expression that corresponds to reversible and adiabatic expansion of an ideal gas is
(a) 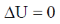
(b) 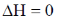
(c) 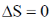
(d) 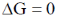
Ans. c
Sol. 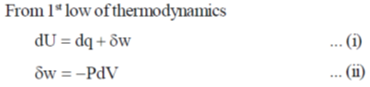
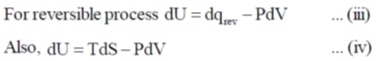
In reversible adiabatic conditions dqrev = 0.
Equation (iii) becomes
20. The major products Y and Z in the following reaction sequence are
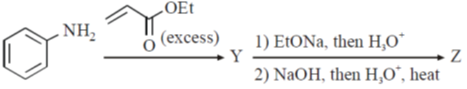
(a) 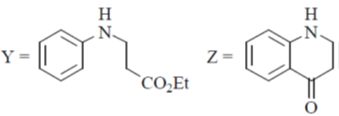
(b) 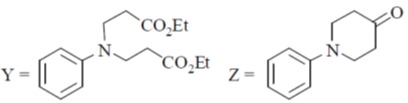
(c) 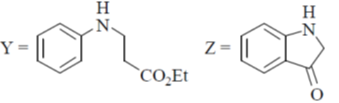
(d) 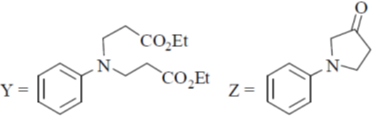
Ans. b
Sol. 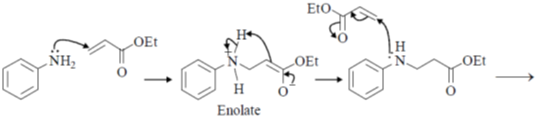
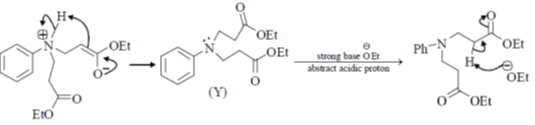
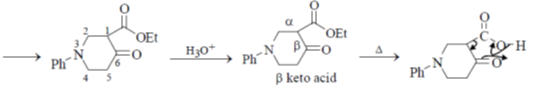
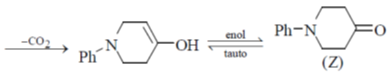
Undergoes decarboxylation on heat through cyclic mechanism to keto product.
Correct answer is (B)
21. Which plot represents a spectrophotometric titration, where the titrant alone absorbs light in the visible region?
(a) 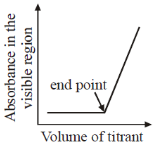
(b) 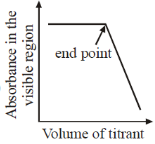
(c) 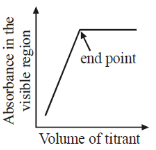
(d) 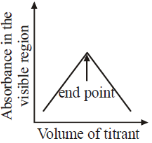
Ans. a
Sol. When titrant is added from burelte into the conical flask; it reacts with titrend and gets eliminated from solution. Fill titrant reacts to titrand, there will be no change in absorbance, when all titrend is consumed.
Now adding titrant, there will be no reaction and hence in conical flask, there is titrant only and its concentrant increase with adding volume therefore absorbance rises after equivalence.
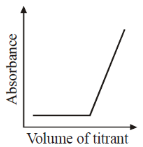
Correct option is (A)
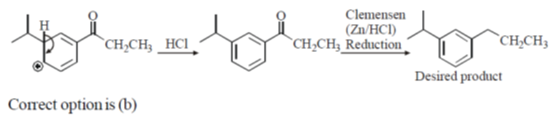
22. The sequence of three steps involved in the following conversion is
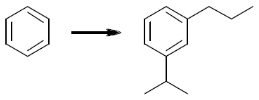
(a) (i) Friedel-Crafts alkylation; (ii) Reduction; (iii) Friedel-Crafts acylation
(b) (i) Friedel-Crafts acylation; (ii) Friedel-Crafts alkylation; (iii) Reduction
(c) (i) Friedel-Crafts acylation; (ii) Reduction; (iii) Friedel-Crafts alkylation
(d) (i) Friedel-Crafts alkylation; (ii) Friedel-Crafts acylation; (iii) Reduction
Ans. b
Sol. Friedel craft acylation of benzene
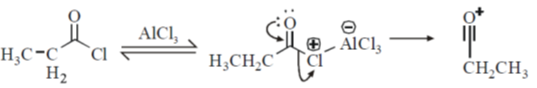
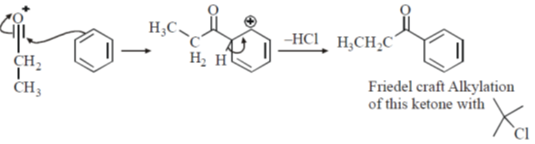

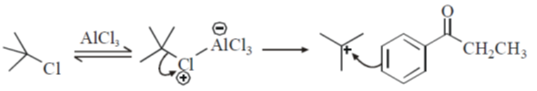
23. 
(a) 
(b) 
(c) 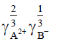
(d) 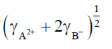
Ans. None
Sol. 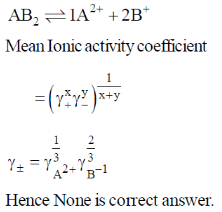
24. Consider the following four xenon compounds: XeF2, XeF4, XeF6 and XeO3 The pair of xenon compounds expected to have non-zero dipole moment is
(a) XeF4 and XeF6
(b) XeF2 and XeF4
(c) XeF2 and XeO3
(d) XeF6 and XeO3
Ans.d
Sol. XeF6 is capped octahedral with partial participation of lone pairs in hybridization which import distorted structure to XeF6 therefore exhbit non zero dipole moment.
In XeO3 there is one lone pair and 3 bond pairs possiessing sp3 hybridization and hence pyramidal shape with non zero dipole moment.
Correct option is (d)
25. The product X in the following reaction sequence is
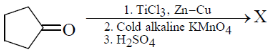
(a) 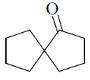
(b) 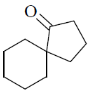
(c) 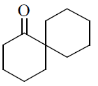
(d) 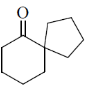
Ans. d
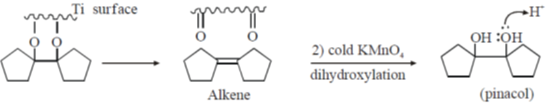
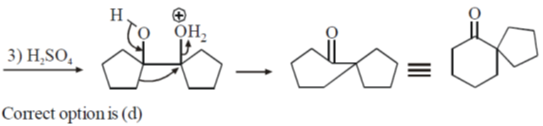
Sol. 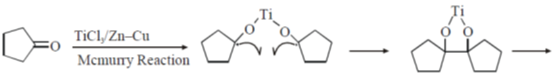
26. Among the dimethylcyclohexanes, which one can be obtained in enantiopure form?
(a) 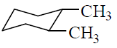
(b) 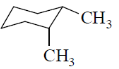
(c) 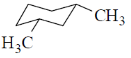
(d) 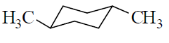
Ans. d
Sol. Due to flunomial behaviour (A) will convert into axial-axial conformation but its equitorial-equitorial conformation is more stable. Hence can be obtained in enantiopure form.
27. The correct order of stability for the following carbocations is
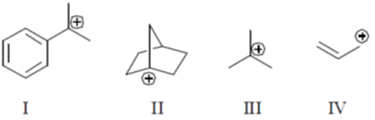
(a) I < III < IV < II
(b) III < II < IV < I
(c) II < IV < III < I
(d) IV < III < I < II
Ans. d
Sol. 
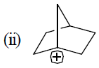 Bridge head carbocation are very unstable because these are too stained and can never become planar. It has high energy vacant sp3 orbital.
Bridge head carbocation are very unstable because these are too stained and can never become planar. It has high energy vacant sp3 orbital.
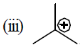 It has 9a hydrogen hyperconjugation stablization therefore more stable than
It has 9a hydrogen hyperconjugation stablization therefore more stable than
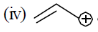 Which enjoys number hyper conjugation but resonance gives some sort of stablization to allylic carbocation.
Which enjoys number hyper conjugation but resonance gives some sort of stablization to allylic carbocation.
28. The major product formed in the following reaction is
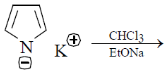
(a) 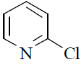
(b) 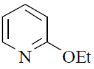
(c) 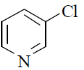
(d) 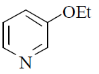
Ans. c
Sol. 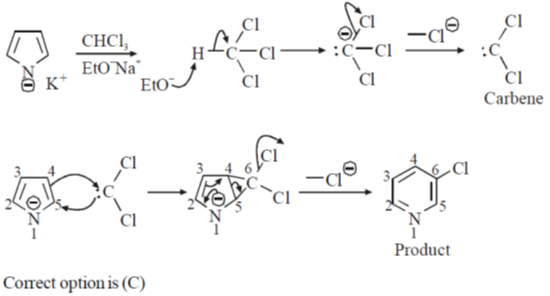
29. 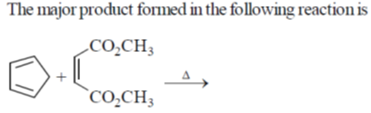
(a) 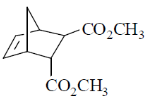
(b) 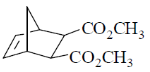
(c) 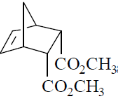
(d) 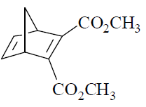
Ans. c
Sol. 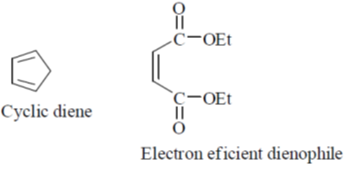
According to Alder rule electron attracting activating group on dienophile will be towards developing 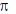 bond in transition state due to secondary orbital stablizing interactions. so endo product is formed.
bond in transition state due to secondary orbital stablizing interactions. so endo product is formed.
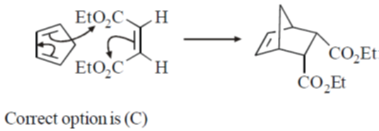
30. The correct order of 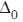 (the octahedral crystal field splitting of d orbitals) values for the following anionic metal complexes is
(the octahedral crystal field splitting of d orbitals) values for the following anionic metal complexes is
(a) 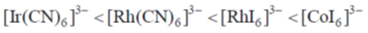
(b) 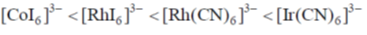
(c) 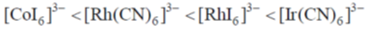
(d) 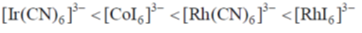
Ans. b
Sol. Ir+3 is a 5d6 metal ion having highest Zeff.complexes with strong 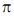 acceptor CN– to provide largest splilting of d orbital in OH environment
acceptor CN– to provide largest splilting of d orbital in OH environment
Rh+3 is 4d6 metal ion shows larger splilting with 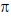 acceptor CN– is compared to
acceptor CN– is compared to 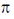 doner I– ligands.
doner I– ligands.
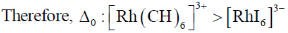
For CO3+ which is 3d6 Is– is weaker field ligand therefore 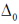 is smallest in
is smallest in 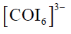
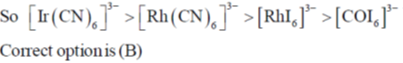
31. Consider the following six solid binary oxides: CaO, Al2O3, PbO, Cs2O, SiO2 and Sb2O3. The pair(s) of ionic oxides is(are)
(a) CaO and Al2O3
(b) CaO and PbO
(c) Cs2O and Al2O3
(d) SiO2 and Sb2O5
Ans. a,b,c
Sol. CaO, Al2O3, PbO, CS2O are ionic oxides as consituents metal ions are very electro positive.
32. Which of the following metal(s) is(are) extracted from its(their) sulfide ore(s) by self-reduction air reduction method?
(a) Cu
(b) Al
(c) Au
(d) Pb
Ans. a, d
Sol. Hg, Ph and Cu are extracted from their PhS and CuS over through air reduction method.
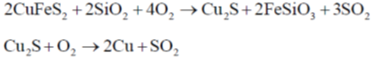
Correct options are (A and D)
33. The correct statement(s) about carbene is(are)
(a) Carbene is a neutral species
(b) Carbene is an intermediate in the Curtius rearrangement
(c) Carbene can insert into both 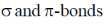
(d) Carbene is generated from amines on reaction with nitrous acid
Ans. a,c
Sol. (A) Carbene is neutral species
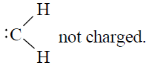
(B) Nitrene is intermediate in curtius rearrangement
(C) Carbene shows N–H/O–H and C–H insertions and also cyclopropanation ring formation with alkenes so can insert in both 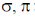 bonds.
bonds.
(D) No carbene can never be generated from amine on reaction with nitrons acid, it gives carbocation, not carbene.
Correct options are (A and C)
34. Choose the correct answer(s) with respect to the magnesium-EDTA titration carried out in the pH range 7 – 10.5, using solochrome black as indicator
(a) Magnesium-indicator complex is more stable than the magnesium-EDTA complex
(b) At the end point, the colour changes from red to blue
(c) After the end point, the colour of the solution is due to the indicator.
(d) pH range of 7 – 10.5 is necessary for observing the specific colour change
Ans. b,c,d
Sol. Correct options are (B, C and D)
35. The correct expression(s) for isothermal expansion of 1 mol of an ideal gas is(are)
(a) 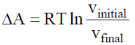
(b) 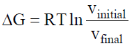
(c) 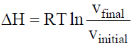
(d) 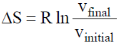
Ans. a,b,d
Sol. 
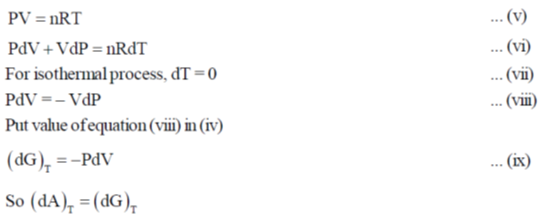
In isothermal expansion of ideal gas, T is constant and as 
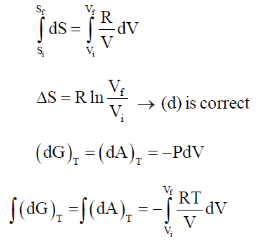
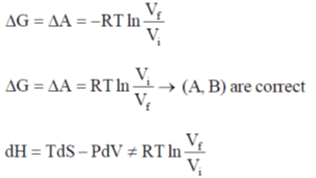
So, option (D) is incorrect
Correct options are (A, B and D)
36. Tetrapeptide(s) that gives (give) the following product on reaction with Sanger's reagent followed by hydrolysis is(are)
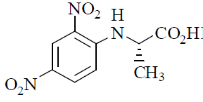
(a) Ala-Gly-Leu-Phe
(b) Asp-Phe-Leu-Pro
(c) Asp-Gly-Tyr-Phe
(d) Ala-Phe-Tyr-Pro
Ans. a,d
Sol. To react with sanger reagent nucleophilic nitrogen is required. Tertiary and amide nitrogens are not nucleophilic. Hence only A and D will produced the above product.
37. The compound(s) that shows (show) positive haloform test is(are)
(a) 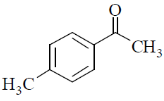
(b) 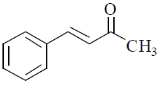
(c) 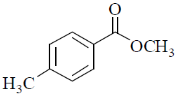
(d) 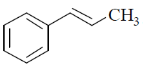
Ans. a,b
Sol. 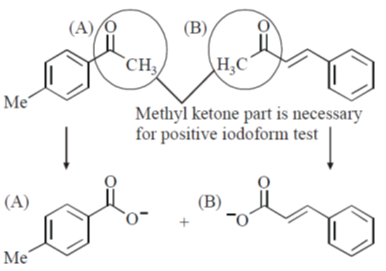
Product of iodoform reaction.
Correct options are (A and B)
38. Which of the following set(s) of quantum numbers is (are) NOT allowed?
(a) n = 3, l = 2, m1 = –1
(b) n = 4, l = 0, m1 = –1
(c) n = 3, l = 3, m1 = –3
(d) n = 5, l = 3, m1 = +2
Ans. b,c
Sol. (B) n = 4 principle quantum number
l = 0 means S orbital and for S orbital 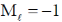 is not valid or allowed.
is not valid or allowed.
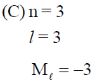
Radial Nodes = n – l – 1 can never be negative
= 3 – 3 – 1
= 3 – 4
= – 1
So (C) is not allowed.
39. In a saturated calomel electrode, the saturation is with respect to
(a) KCl
(b) Hg2Cl2
(c) HgCl2
(d) AgCl
Ans. a,b
Sol. Saturated calomel electrode is composed of saturated solution of KCl and paste of Hg2Cl2. Sparingly soluble salt.
Correct options are (A and B)
40. On reaction with NaNO2 and HCl, which of the following amino alcohol(s) will yield compound P?
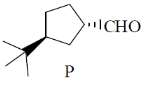
(a) 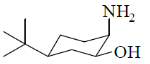
(b) 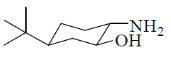
(c) 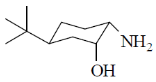
(d) 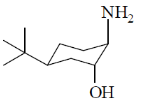
Ans. b,c
Sol. 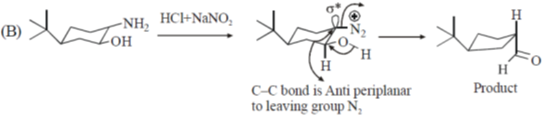
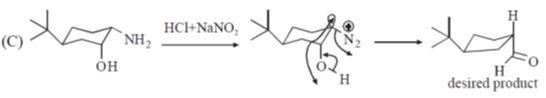
 bond is Anti periplanar to
bond is Anti periplanar to 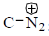 bond therefore easily / smoothly donates this electron density into vacant
bond therefore easily / smoothly donates this electron density into vacant 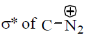 so ring contraction assisted by
so ring contraction assisted by 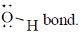
Correct options are (B and C)
41. Among the following hydrocarbon(s), how many of them would give rise to three groups of proton NMR peaks with 2:2:3 integration ratio?
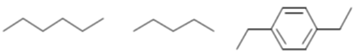
Ans. 2
Sol. 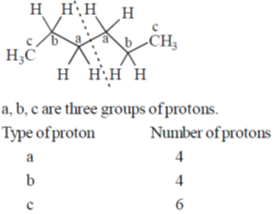
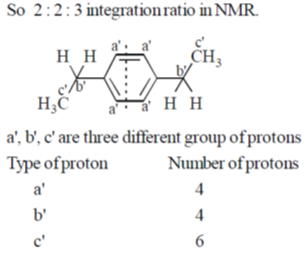
So, 2 : 2 : 3 integration ratio in NMR.
Correct answer is (2)
42. The nuclear spin quantum number (I) of a nucleus is 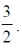 When placed in an external magnetic field. the number of possible spin energy states it can occupy is __________.
When placed in an external magnetic field. the number of possible spin energy states it can occupy is __________.
Ans. 4
Sol. No. of possible nuclear energy state for a nuclei whose nuclear quantum number, 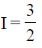 is given by 2I + 1
is given by 2I + 1
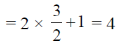
This nuclei will have 4 nuclear spin energy levels which are apparently available for occupation.
So answer is 4.
43. The number of possible isomers for 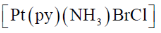 is _______ (py is pyridine)
is _______ (py is pyridine)
Ans. 3
Sol. 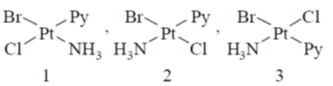
3 isomers are possible for [Pt(Py)BrCl].
44. The number of stereoisomers possible for the following compound is __________.
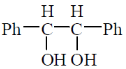
Ans. 3
Sol. No. of stereoisomer for molecule having internal plane of symmetry / meso compound.
n = number of chiral centers = 2
no. of stereoisomer = 2n – 1
= 22 – 1
= 4 – 1
= 3.
45. Assuming ideal gas behavior, the density of O2 gas at 300 K and 1.0 atm is ______ gL–1 (rounded up to two decimal places).
[R = 0.082 L atm mol–1 K–1, molar mass of O2 = 32]
Ans. 1.3
Sol. 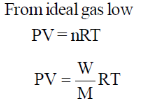
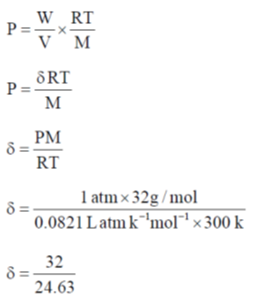
Correct answer is (1.3)
46. The time for 50% completion of a zero order reaction is 30 min. Time for 80% completion of this reaction is _______ min.
Ans. 48
Sol. For zero order reaction, concentration – time relation
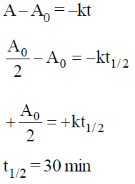

47. The volume of 0.3 M ferrous ammonium sulphate solution required for the completion of redox titration with 20 mL of 0.1 M potassium dichromate solution is _______ mL.
Ans. 40
Sol. 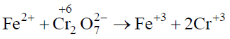
No. of g equivalents of Fe2+ = No. of g equivalents of Cr2O72–
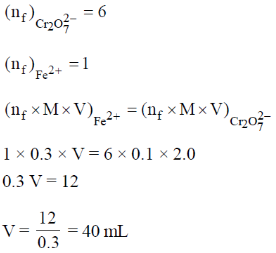
48. The value of Cv for 1 mol of N2 gas predicted from the principle of equipartition of energy, ignoring vibrational contribution, is _______ J K–1 mol–1 (rounded up to two decimal places).
[R = 8.3 JK–1 mol–1]
Ans. 20.75
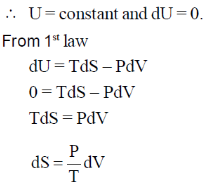
Sol. According to low of equipartition of energy each coordinate axis is associated with 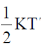 energy for rotational and transitional degrees of freedom for N2 linear molecule
energy for rotational and transitional degrees of freedom for N2 linear molecule
There is 2 rotational degree of freedom 3 translational degree of freedom
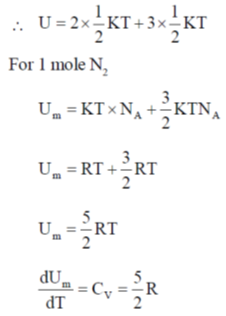

49. The number of hydrogen bond(s) present in a guanine-cytosine base pair is _______.
Ans. 3
Sol. 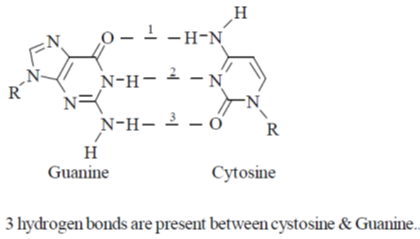
50. 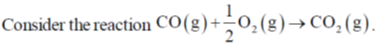
The value of 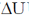 for the reaction at 300 K is –281.8 kJ mol–1. The value of
for the reaction at 300 K is –281.8 kJ mol–1. The value of 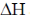 at same temperature is ________ kJ mol–1 (rounded up to the first decimal place).
at same temperature is ________ kJ mol–1 (rounded up to the first decimal place).
[R = 8.3 J K–1 mol–1]
Ans. -283.0
Sol. 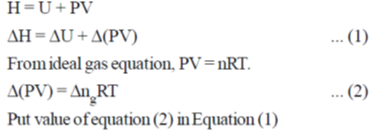
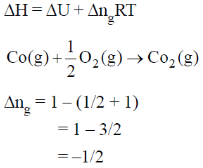
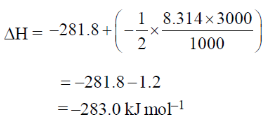
51. 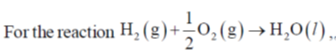 the following information is given T = 300 K
the following information is given T = 300 K
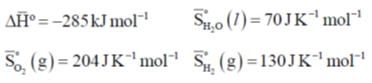

Ans. 788
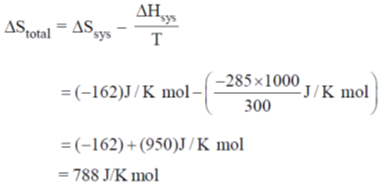
Sol. 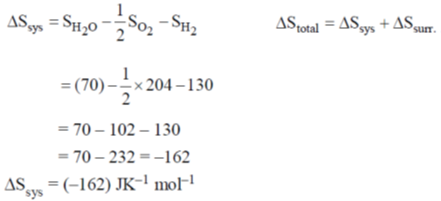
52. How many of the following interhalogen species have 2 lone pairs of electrons on the central atom?
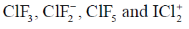
Ans. 2
Sol. 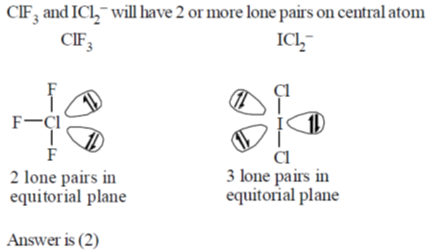
53. The solubility of PbI2 in 0.10 M KI(aq) is ______ × 10–7 M (rounded up to two decimal places).
[The solubility product, 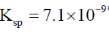 ]
]
Ans. 7.1
Sol. 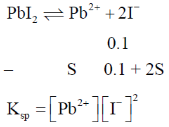

54. The number of compounds having S-configuration among the following is ______.
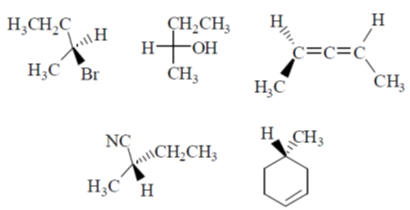
Ans. 4
Sol. 
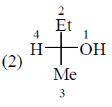 Since least priority is at horizontal, therefore Anticlockwise S configuration will revert to R configuration.
Since least priority is at horizontal, therefore Anticlockwise S configuration will revert to R configuration.
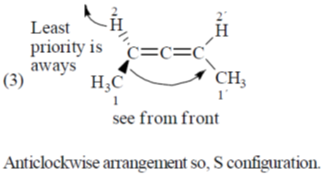
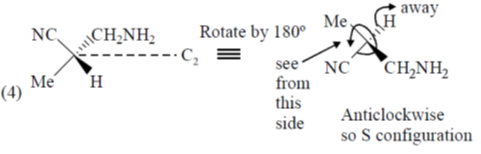
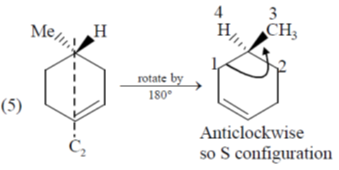
Correct answer is (4)
55. The magnitude of crystal field stabilization energy (CFSE) of octahedral 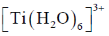 complex is 7680 cm–1. The wavelength at the maximum absorption
complex is 7680 cm–1. The wavelength at the maximum absorption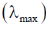 of this complex is ______ nm (rounded up to the nearest integer).
of this complex is ______ nm (rounded up to the nearest integer).
Ans. 1
Sol. Electronic contant Ti3+ 3d'45º
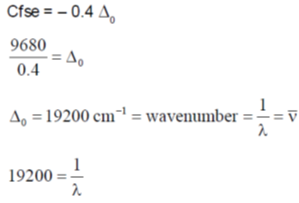
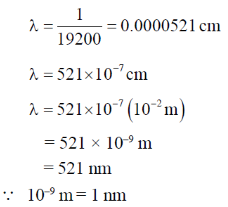
56. The electron of a hydrogen atom is in its nth Bohr orbit having de Broglie wavelength of 13.4 Å. The value of n is _________ (rounded up to the nearest integer).
[Radius of nth Bohr orbit = 0.53 n2 Å, 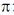 = 3.14]
= 3.14]
Ans. 4
Sol. 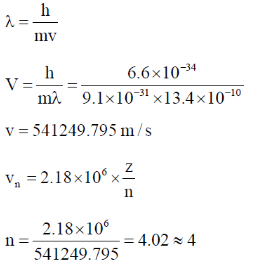
57. Elemental analysis of an organic compound contaming C, H and O gives percentage composition: C : 39.9% and H : 6.7%. If the molecular weight of the compound is 180, the number of carbon atoms present in the molecule is ________.
Ans. 6
Sol. In given organic compound % of C is 39.9 therefore fraction of C is 0.399.
So, weight of C in given compound = 0.399 × Molecular weight of compound
= 0.399 × 180
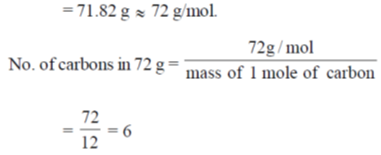
58. The emf of a standard cadmium cell is 1.02 V at 300 K. The temperature coefficient of the cell is 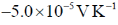 . The value of
. The value of  for the cell is _______ kJ mol–1 (rounded up to two decimal places).
for the cell is _______ kJ mol–1 (rounded up to two decimal places).
[1 F = 96500 C mol–1]
Ans. –199.75
Sol. 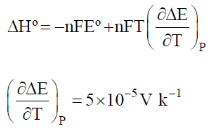
Eº = 1.02 V;T = 300 K
For cadmium cell, number of electron exchanged n = 2
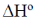 = –2 × F × 1.02 + 2F × 300 (–5 × 10–5)
= –2 × F × 1.02 + 2F × 300 (–5 × 10–5)
= –2.04 F – 0.03 F
= –2.07 F
= –2.07 × 96500
= –199755 J mol–1
= –199.75 kJ mol–1
59.For H2 molecule, the fundamental vibrational frequency 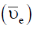 in wavenumbers can be taken as
in wavenumbers can be taken as 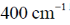 . the zero-point energy of the molecule is _______ kJ mol–1 (rounded up to two decimal places).
. the zero-point energy of the molecule is _______ kJ mol–1 (rounded up to two decimal places).
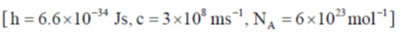
Ans. 26.13
Sol. 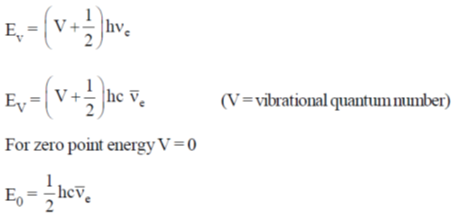
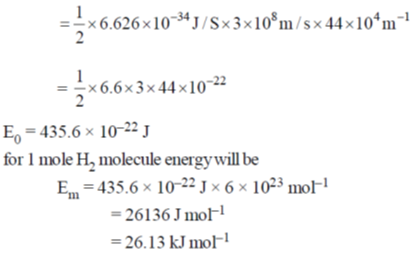
60. 24Na decays to one-fourth of its initial amount in 29.8 hours. Its decay constant is ______ hour–1 (rounded up to four decimal places).
Ans. 0.0465
Sol. For 1st order reaction