IIT JAM Chemistry 2017
Previous Year Question Paper with Solution.
1. Catalytic hydrogenation of the following compound produces saturated hydrocarbon(s). The number of stereoisomer(s) formed is

(a) 1
(b) 2
(c) 3
(d) 4
Ans. c
Sol. 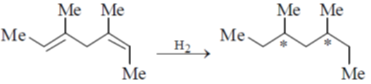
Total number of stereocentres in product is two and it contains symmetry hence total number of stereoisomers formed is calculated by

Hence (c) is correct answer.
2. The number of normal modes of vibration in naphthalene is
(a) 55
(b) 54
(c) 48
(d) 49
Ans. c
Sol. 
3. In the following sequence of reactions, the overall yield (%) of O is

(a) 61
(b) 85
(c) 74
(d) 68
Ans. a
Sol. 
Hence (A) is correct answer.
4. The correct order of rate of solvolysis for the following compound is
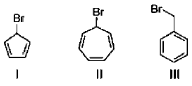
(a) III > II > I
(b) II > I > III
(c) III > I > II
(d) II > III > I
Ans. d
Sol. Rate of solvolysis depends upon stability of carbocation formed in the SN1 solvolysis reaction.
Hence,

Stability of carbocation follows 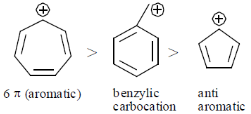
Hence correct answer is (D) II > II > I
5. The correct order of the boiling points of the compounds is
(a) CH4 > SiH4 > SnH4 > GeH4
(b) SiH4 > CH4 > GeH4 > SnH4
(c) SnH4 > GeH4 > CH4 > SiH4
(d) SnH4 > GeH4 > SiH4 > CH4
Ans. d
Sol. In a group going from up to down molecular weight increases hence boiling point also increases.
Hence correct sequence of boiling point is CH4 < SiH4 < GeH4 < SnH4
Hence Correct answer is (d).
6. The compounds having C3-axis of symmetry are
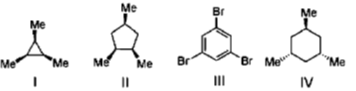
(a) I, III and IV
(b) I, II and III
(c) I and III
(d) III and IV
Ans. c
Sol. 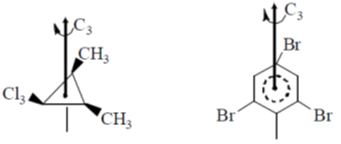
these two compounds contains C3 symmetry.
7. In the following Latimer diagram, the species the undergoes disproportionation reaction is
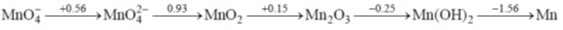
(a) MnO42–
(b) MnO43–
(c) Mn2O3
(d) Mn(OH)2
Ans. b
Sol. 
For MnO43– its standard reduction potential is greater value than the standard oxidation potential hence it will undergo dispropotionation reaction due to overall standard potential is positive

Hence it will undergo disproportionation reaction.
8. A yellow precipitate is formed upon addition of aqueous AgNO3 to a solution of
(a) phosphite
(b) pyrophosphate
(c) metaphosphate
(d) orthophosphate
Ans. d
Sol. 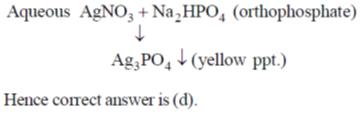
9. A straight line having a slope of 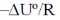 is obtained in a plot between
is obtained in a plot between
(a) ln Kp versus T
(b) ln (KC) versus T
(c) ln (KP) versus 1/T
(d) ln (KC) versus 1/T
Ans. d
Sol. 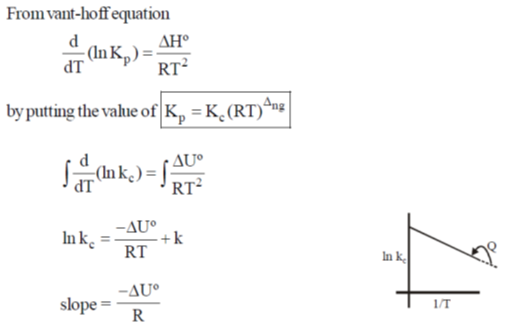
10. The number of degrees of freedom of liquid water in equilibrium with ice is
(a) 0
(b) 1
(c) 2
(d) 3
Ans. b
Sol. 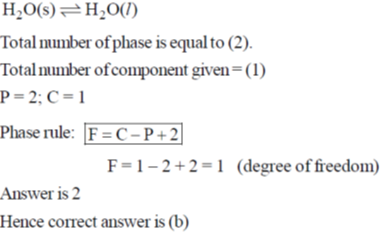
11. The number of proton NMR signals for the compounds P and Q, respectively, is
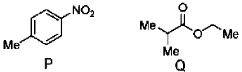
(a) 3 and 4
(b) 3 and 5
(c) 4 and 3
(d) 5 and 4
Ans. a
Sol. 
Number of proton NMR signals = total number of chemical nonequivalent protons
Hence in (P) total type of protons are three and in (Q) it is four.
Hence correct answer is (A).
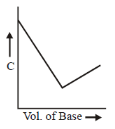
12. In a typical conductometric titration of a strong acid with a weak base, the curve resembles
(a) 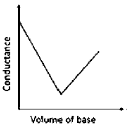
(b) 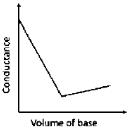
(c) 
(d) 
Ans. a
Sol. Conductometric titration of strong acid with weak base.
Before addition of weak base
Initially strong acid is present which have high conductivity H+ present
 .
.
Before equivalence point:
Highly conductive H+ are replaced by weakly conductive NH4+
After Equivalence point
Conductance increases but not very much because of number of ions increase from the dissociation of NH4OH into NH4+ & OH–.
13. The correct order of enthalpy of hydration for the transition metal ions is
(a) Cr2+ > Mn2+ > Co2+ > Ni2+
(b) Ni2+ > Co2+ > Mn2+ > Cr2+
(c) Ni2+ > Co2+ > Cr2+ > Mn2+
(d) Cr2+ > Mn2+ > Ni2+ > Co2+
Ans. c
Sol. 
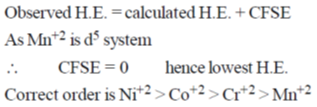
14. The product R in the following reaction is
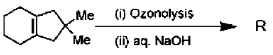
(a) 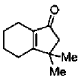
(b) 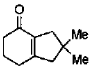
(c) 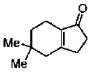
(d) 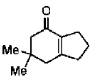
Ans. d
Sol. 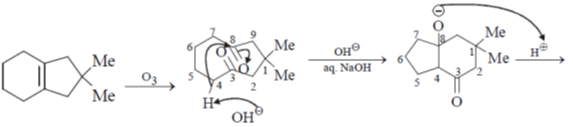

15. 
(a) circle
(b) ellipse
(c) bell-shaped curve
(d) hyperbola
Ans. d
Sol. 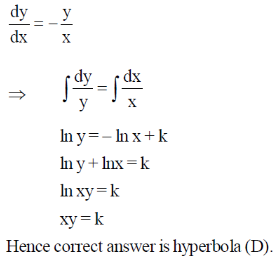
16. The coordination number of Al in crystalline AlCl3 and liquid AlCl3, respectively is
(a) 4 and 4
(b) 6 and 6
(c) 6 and 4
(d) 3 and 6
Ans. c
Sol. 
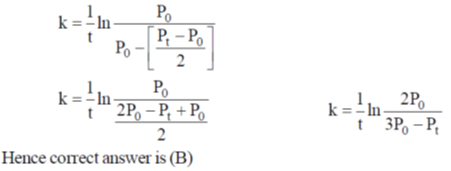
17. For a first order reaction 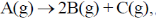 the rate constant is terms of initial pressure (p0) and pressure at time t(pt) is given by
the rate constant is terms of initial pressure (p0) and pressure at time t(pt) is given by
(a) 
(b) 
(c) 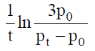
(d) 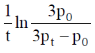
Ans. b
Sol. 
18. The following conversion is carried out using

(a) hydroboration oxidation followed by Jones oxidation
(b) Wacker oxidation followed by haloform reaction
(c) oxymercuration-determination followed by Jones oxidation
(d) ozonolysis followed by haloform reaction
Ans. b
Sol. 
Hence correct answer is B.
19. In the following reactions, the major products E and F, respectively are
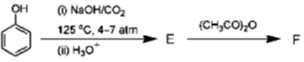
(a) 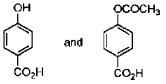
(b) 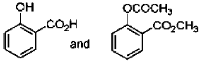
(c) 
(d) 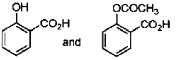
Ans. d
Sol. 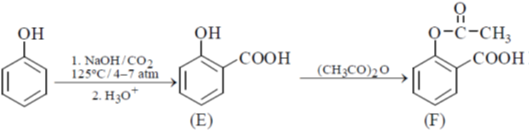
Correct option is (d)
20. For a particle in one-dimensional box of length L with potential energy V(x) = 0 for L > x > 0 and V(x) =  for x > L and x < 0 an acceptable wave function consistent with the boundary conditions is (A, B, C and D are constants)
for x > L and x < 0 an acceptable wave function consistent with the boundary conditions is (A, B, C and D are constants)
(a) 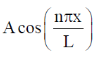
(b) B(x + x2)
(c) Cx3 (x – L)
(d) 
Ans. c
Sol. For particle in 1-D box of length 0 to l acceptable wave function is
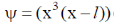
only this wave function satisfy boundary condition because when x = 0, 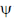 = 0 also when n = l,
= 0 also when n = l,  = 0.
= 0.
21. Ionisation energy of hydrogen atom is ground state is 13.6 eV. The energy released (in eV) for third member of Balmer series is
(a) 13.056
(b) 2.856
(c) 0.967
(d) 0.306
Ans. c
Sol. 
22. The metal ion (M2+) in the following reaction is

(a) Mn2+
(b) Fe2+
(c) Cd2+
(d) Cu2+
Ans. d
Sol. 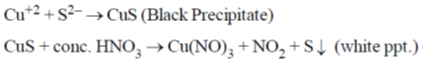
23. Among the following compounds, the pair of enantiomers is

(a) I and IV
(b) I and III
(c) II and III
(d) III and IV
Ans. b
Sol. 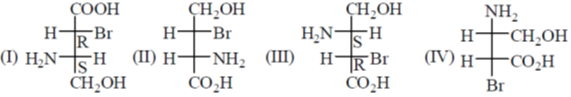
I & II are structural isomers.
I & III are mirror image hence pair of enantiomers
Hence Correct answer is (B)
24. Value of the given determinant is

(a) -12
(b) 0
(c) 6
(d) 12
Ans. a
Sol. 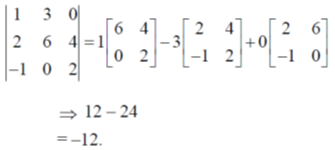
25. The correct order of wavelength of absorption 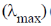 of the Cr-complexes is (en = ethylenediamine)
of the Cr-complexes is (en = ethylenediamine)
(a) [CrF6]3– > [Cr(H2O)6]3+ > [Cr(en)3]3+ > [Cr(CN)6]3–
(b) [Cr(H2O)6]3+ > [CrF6]3– >[Cr(en)3]3+ > [Cr(CN)6]3–
(c) [Cr(CN)6]3– > [Cr(en)3]3+ > [Cr(H2O)6]3+ > [CrF6]3–
(d) [Cr(en)3]3+ > [Cr(CN)6]3– > [Cr(H2O)6]3+ > [CrF6]3–
Ans. a
Sol. Wavelength of absorption 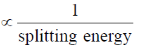
Also, splitting energy depends on field on ligand, stronger the field of ligand, more will be splitting energy.
Order of ligand strength CN– > en > H2O > F

[CrF6]3– > [Cr(H2O)6]+3 > [Cr(en)3]+3 > [Cr(CN)6]+3.
26. In the following reaction, the major product T is
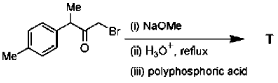
(a) 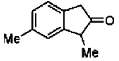
(b) 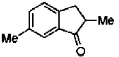
(c) 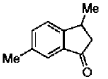
(d) 
Ans. c
Sol. 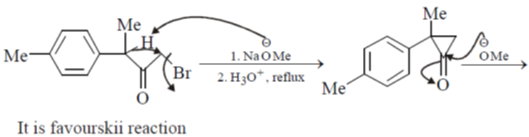
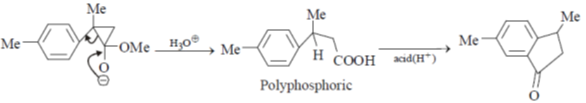
Hence correct answer is (C).
27. The homogeneous catalyst used in water-gas shift reaction is
(a) PdCl2
(b) Cr2O3
(c) [RhCl(PPh3)3]
(d) [RuCl2(bipyridyl)2]
Ans. d
Sol. The homogeneous catalyst used in water gas shift reaction is RuCl2 (bipyridyl)2.
28. The major product S of the following reaction is
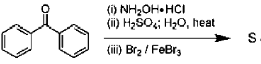
(a) 
(b) 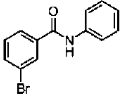
(c) 
(d) 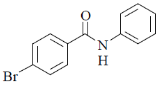
Ans. c
Sol. 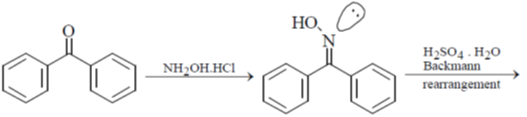
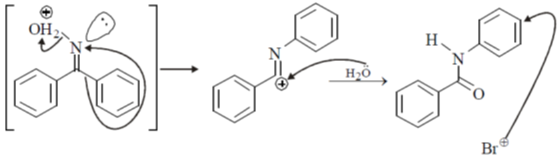
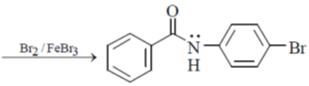
due to +M effect of nitrogen ring linked with nitrogen give electrophilic substitution on that ring.
Hence both ortho and para product forms but para one is the major product hence (C) is the correct answer.
29. Nitrosyl ligand binds to d-metal atoms in linear and bent fashion and behaves, respectively, as
(a) NO+ and NO+
(b) NO+ and NO–
(c) NO– and NO–
(d) NO– and NO+
Ans.
Sol. When NO binds in linear fashion, it acts as 3e– donor, the formal charge is +1 and shows  interaction (NO+)
interaction (NO+)
When NO binds in bent fashion, it acts as 1e– donor, formal charge is –1 and show only  interaction (NO–)
interaction (NO–)
30. The correct set of reagents for the following conversion is

(a) (i) NaNH2/liq. NH3; (ii) NaNO2/dil. HCl; (iii) CuCN, heat
(b) (i) HNO3/H2SO4; (ii) Zn/HCl; (iii) NaNO2/dil. HCl; (iv) CuCN, heat
(c) (i) Mg/ether, H2O+; (ii) (EtO)2CO; (iii) NH4OH; (iv) PCl5
(d) (i) Mg/ether, H2O+; (ii) HNO3/H2SO4; (iii) NaNO2/dil. HCl; (iv) CuCN, heat
Ans. a
Sol. 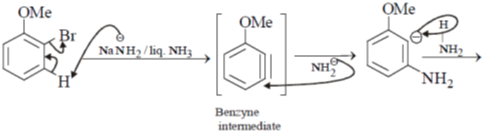
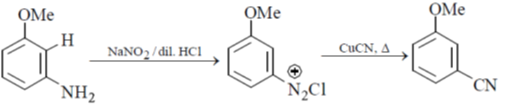
Hence answer (A) is correct
31. The indicator(s) appropriate for determination of end point in the titration of a weak acid with a strong base is/are
(a) Phenolphthalein
(b) thymol blue
(c) bromophenol blue
(d) methyl orange
Ans. a,b
Sol. Titration of weak acid with strong base

Only those indicators having indicator range lie within steep curve can be used for titration
 phenolpthalein and thymol blue are suitable indicators.
phenolpthalein and thymol blue are suitable indicators.
32. IR active molecule(s) is/are
(a) CO2
(b) CS2
(c) OCS
(d) NO2
Ans. a,b,c
Sol. For a molecule to IR active must have dynamic dipole moment.
Except homodiatomic molecule all molecule are IR active.
Out of CO2, CS2, OCS, N2 only N2 is IR active.

Hence correct answer are (a, b, c)
33. Among the following the correct statement(s) is/are
(a) Guanine is a purine nucleobase
(b) Glycine and proline are achiral amino acids
(c) DNA contains glycosidic bonds and pentose sugars
(d) Sucrose is a non-reducing sugar
Ans. a,c,d
Sol. 
Because it contains pyrimidine ring fused to an imidazole ring hence it is purine nucleobase.

Because glycine does not contains chiral carbon hence it is achiral aminoacids but proline is chiral amino acid.
(C) DNA contains pentose sugar joined together by phosphate groups and it contains glycosidic bonds.
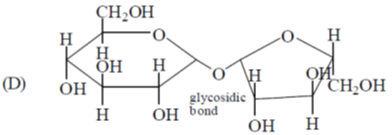
Sucrose contains glycosidic bond between its anomeric carbons thus can not converted to an open chain form with an aldehyde group.
Hence A, C, D are correct answers.
34. Among the following, the species having see-saw shape is/are
(a) SF4
(b) XeF4
(c) ClF4+
(d) 
Ans. a, c
Sol. SF4
Steric Number 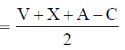
A = Anionic charge, C = Cationic charge, V = Valence electron of central atom, X = monovalent atoms
Steric Number 
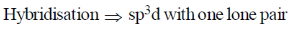
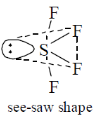
XeF4
Steric Number 
sp3d2 = octahedral geometry with two lone pair


ClF4–
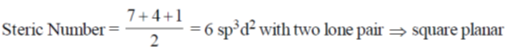
Hence SF4 and ClF4+ have see-saw shape.
35. The following conversion(s) is/are example(s) of

(a) oxy-Cope rearrangement
(b) Sigmatropic rearrangement
(c) Claisen rearrangement
(d) Pericyclic reaction
Ans. b,c,d
Sol. 
(B) [3,3]-sigmatropic reaction: A intramolecular process where one sigma bond is changed to another sigma bond.
(C) Claisen rearrangement because it includes allyl-vinyl ether.
(D) Pericyclic reaction since sigmatropic reaction is a type of pericyclic reaction.
36. Jahn-Teller distortion is/are observed in octahedral complexes with d-electron configuration of
(a) d5-high spin
(b) d5-low spin
(c) d6-high spin
(d) d6-low spin
Ans. b,c
Sol. JTD is observed whenever their is electronic degeneracy or t2g / eg are unsymmetrically filled.
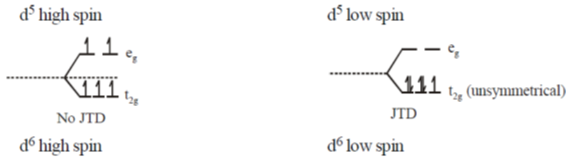

Hence d5(HS) and d6(LS) are correct answer.
37. Wave nature of electromagnetic radiation is observed in
(a) diffraction
(b) interference
(c) photoelectric effect
(d) Compton scattering
Ans. a,b
Sol. Wave nature is observed in diffraction and interference.
38. The incorrect statement(s) among the following is/are
(a) 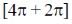 cycloaddition reactions are carried out in presence of light
cycloaddition reactions are carried out in presence of light
(b) 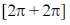 cycloaddition reaction between a keto group and an alkene is photochemically allowed.
cycloaddition reaction between a keto group and an alkene is photochemically allowed.
(c) 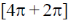 cycloaddition reactions are thermally allowed
cycloaddition reactions are thermally allowed
(d) Transoid dienes undergo Diels-alder reactions
Ans. a,d
Sol. 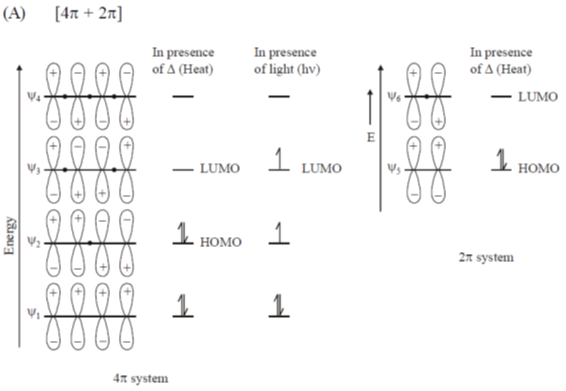
Since reaction occurs between HOMO & LUMO

Hence, statement A is wrong.
Statement (B) is the condition for Patrno Buchi reaction in which a keto group and an alkene is reacted in photochemical condition.
So, it is a correct statement
Statement (C):  cycloaddition reactions are thermally allowed.
cycloaddition reactions are thermally allowed.
So it is a correct statement
Statement (D): Transoid dienes undergo Diels-Alder Reaction.
So it is a incorrect statement.

Here cycloaddition can't possible because overlapping will not be effective.
Correct answer is A, D.
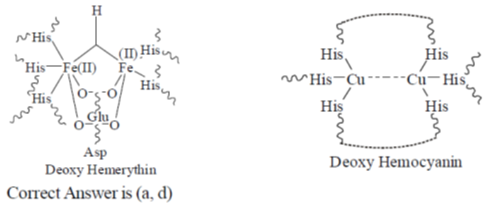
39. The "heme" containing protein(s) is/are
(a) cytochrome C
(b) hemocyanin
(c) hemerythrin
(d) myoglobin
Ans. a,d
Sol. 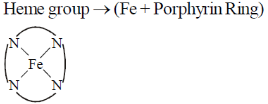
Myoglobin and cytochrome-C contain Heme group. Whereas Hemocyanin and hemerythin are non-Heme protein.
40. Intensive variable(s) is/are
(a) temperature
(b) volume
(c) pressure
(d) density
Ans. a,c,d
Sol. Intensive variable are temperature, pressure and density. Because these properties are independent of bulk of system.
41. The number of unpaired electron(s) in K2NiF6 is _______
Ans. 0
Sol. K2NiF6
When Ni+4, F– will act as strong field ligand 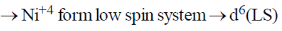
42. Among the following, the number of aromatic compound(s) is ______
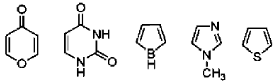
Ans. 4
Sol. 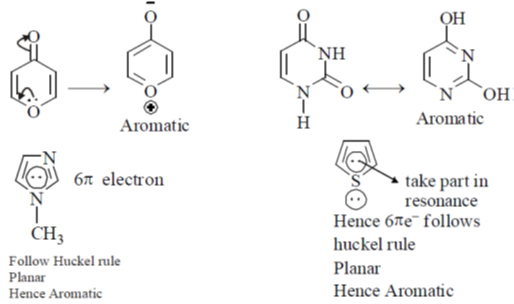
43. The maximum number of dipeptides that could be obtained by reaction of phenylalanine with leucine is ______
Ans. 2
Sol. 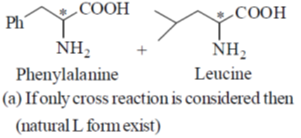

(b) If self reaction and cross reaction both considered then the answer will be four.
(c) If D and L both form considered (not natural form) then answer will be eight.
44. The number of S-S bond(s) in tetrathionate ion is _____
Ans. 3
Sol. 
45. For a cell reaction, 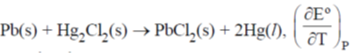 s 1.45 × 10–4 VK–1. The entropy change (in J mol–1 K–1) for the reaction is ______
s 1.45 × 10–4 VK–1. The entropy change (in J mol–1 K–1) for the reaction is ______
Ans. 27.98
Sol. 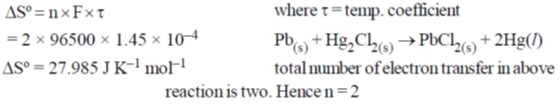
Hence correct answer is 27.98.
46. For a reaction 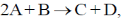 if rate of consumption of A is 0.1 mol L–1 s–1 , the rate of production of C (in mol L–1 s–1) is _____
if rate of consumption of A is 0.1 mol L–1 s–1 , the rate of production of C (in mol L–1 s–1) is _____
Ans. 0.05
Sol. 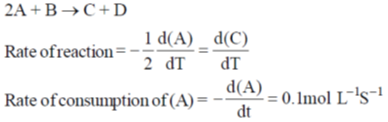
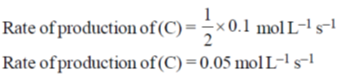
Hence correct answer is 0.05.
47. The number of isomeric structures of di-substituted borazine (B3N3H4X2) is ______
Ans. 4
Sol. For B3N3H4X2
Total isomers possible are
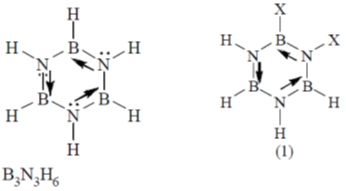

hence correct answer is 4
48. At an operating frequency of 350 MHz, the shift (in Hz) of resonance from TMS (tetramethylsilane) of a proton with chemical shift of 2 ppm is _____
Ans. 700
Sol. 
Shift (Hz) = 700 Hz
49. The number of reducing sugars among the following is ______
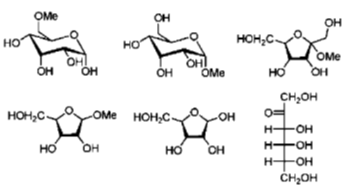
Ans. 3
Sol. Condition for reducing sugar
 Monosaccharide that have a aldehyde group. They can act as reducing sugar by converting it to carboxylic group.
Monosaccharide that have a aldehyde group. They can act as reducing sugar by converting it to carboxylic group.
 this keto group can be converted into aldehyde group under basic condition.
this keto group can be converted into aldehyde group under basic condition.
Rest of all structure will not able to give sugar with aldehyde group
Hence answer is 3.
50. At 298 K and 1 atm, the molar enthalpies of combustion of cyclopropane and propene are –2091 kJ mol–1 and –2058 kJ mol–1, respectively. The enthalpy change (in kJ mol–1) for the conversion of one mole of propene to one mole of cyclopropane is _______
Ans. At 298 K & 1 atm
Sol. 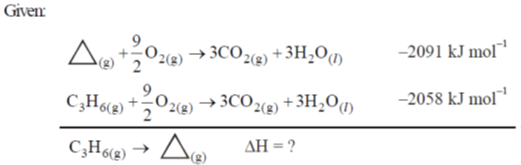
by reversing the first reaction and adding in second reaction we will get the desired reaction

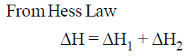
= (2091 – 2058) kJ mol–1 = 33 kJ mol–1
51. The standard reduction potentials of Ce4+/Ce3+ and Fe3+/Fe2+ are 1.44 and 0.77V, respectively. The log10 K (K is the equilibrium constant) value for the following reaction is ______
(final answer should be rounded off to two decimal places)

Ans. 11.32
Sol. 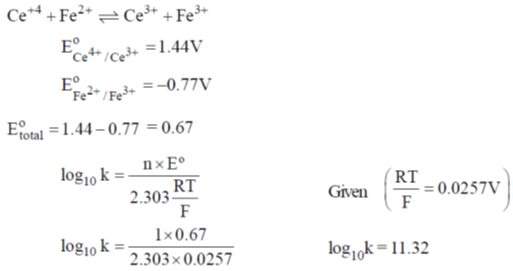
Hence correct answer is 11.32.
52. A radioactive element undergoes 80% radioactive decay in 300 min. The half-life for this species in minutes is ______
Ans. 129.15
Sol. 
Hence correct answer is 129.15.
53. The amount of bromine (atomic wt. = 80) required (in gram) for the estimation of 42.3 g of phenol (molecular wt. = 94 g mol–1) is _______
Ans. 216
Sol. 
3 moles of bromine is required for one mol phenol.

Hence number of moles of Br2 required = 3 × 0.45 mol
amount of bromine required = 3 × 0.45 × 160 g = 216 g
54. The total number of pair of enantiomers possible with molecular formula C5H12O is _____
Ans. d
Sol. 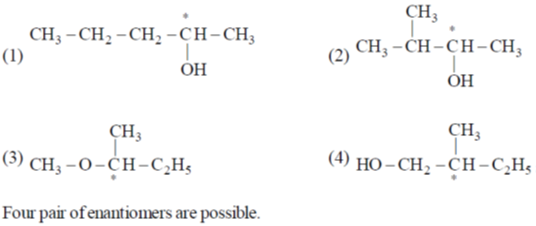
55. Silver crystallizes in a face-centered cubic lattice. The lattice parameter of silver (in picometer) is _____
Ans. 408.6
Sol. 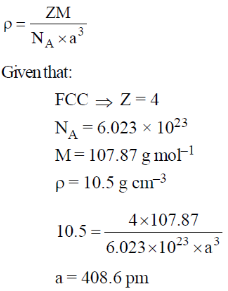
56. A vessel contains a mixture of H2 and N2 gas. The density of this gas mixture is 0.2 g L–1 at 300 K and 1 atm. Assuming that both the gases behave ideally, the mole fraction of N2(g) in the vessel is _______
(final answer should be rounded off to two decimal places)
[Given: R = 0.082 L atm mol–1 K–1, atomic wt. of hydrogen = 1.0 and atomic wt. of nitrogen = 14.0]
Ans. 0.11
Sol. 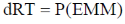
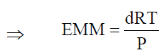 where EMM = Effective molar mass of gaseous mixture.
where EMM = Effective molar mass of gaseous mixture.
Given that: d = 0.2 g L–1; T = 300 K; P = 1 atm; R = 0.082 L atm mol–1 k–1

57. Consider an isothermal reversible compression of one mole of an ideal gas in which the pressure of the system is increased from 5 atm to 30 atm at 300 K. The entropy change of the surroundings (in J K–1) is _______
(final answer should be rounded off to two decimal places)
[Given: R = 8.314 J mol–1 K–1]
Ans. 14.90
Sol. Reversible isothermal compression of one mole of ideal gas from 0.5 atm to 30 atm at 300 K


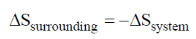 for isothermal reversible compression of ideal gas.
for isothermal reversible compression of ideal gas.

58. In 200 g of water, 0.01 mole of NaCl and 0.02 mole of sucrose are dissolved. Assuming solution to be ideal, the depression in freezing point of water (in ºC) will be ______
(final answer should be rounded off to two decimal places)
[Given: Kf(H2O) = 1.86 K kg mol–1]
Ans. 0.372
Sol. 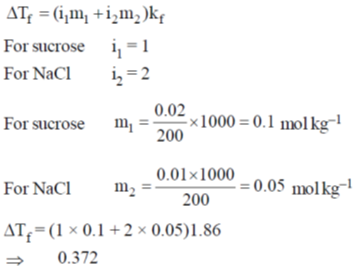
59. The adsorption of a gas follows the Langmuir isotherm with K = 1.25 kPa–1 at 25ºC. The pressure (in Pa) in which surface coverage is 0.2 is ______
Ans. 200
Sol. For langmuir isotherm
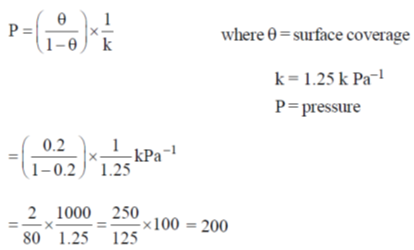
Hence correct answer is 200.
60. The separation of 123 planes (in nm) in an orthorhombic cell with a = 0.25 nm, b = 0.5 nm and c = 0.75 nm is ______
(final answer should be rounded off to two-decimal places)
Ans. 0.144
Sol. 
