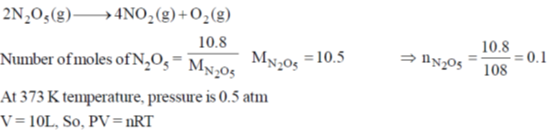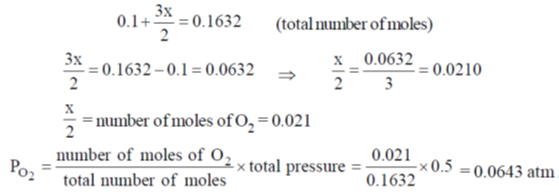IIT JAM Chemistry 2016
Previous Year Question Paper with Solution.
1. The correct order of pKa for the following compounds is
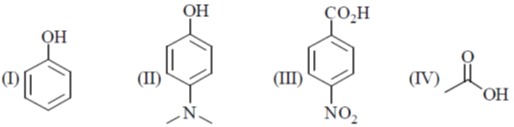
(a) II > I > III > IV
(b) II > I > IV > III
(c) III > IV > I > II
(d) IV > II > I > III
Ans. b
Sol. 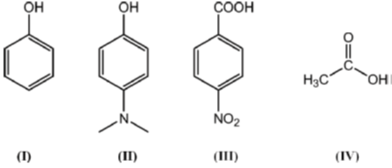
• Electron withdrawing group increases the acidic character
• Electron donating group decreases the acidic behaviour
• Benzoic acid is more acidic as compared to acetic acid, which in turn is more acidic as compared to phenol.
Thus order of ocidic behaviour will be III > IV > I > II
Hence, correct order of pKa will be III < IV < I < II
Correct Option is (B)
2. The major product formed in the following reaction is
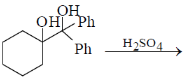
(a) 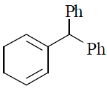
(b) 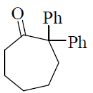
(c) 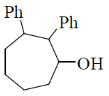
(d) 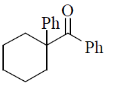
Ans. b
Sol. 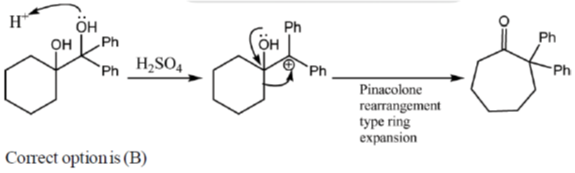
3. The mechanism of the following transformation involves
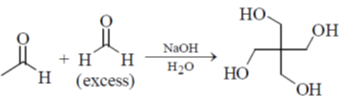
(a) Aldol reaction and Cannizzaro reaction
(b) Aldol reaction and Claisen-Schmidt reaction
(c) Knoevenagel condensation and Cannizzaro reaction
(d) Stobbe condensation and Cannizzaro reaction
Ans. a
Sol. 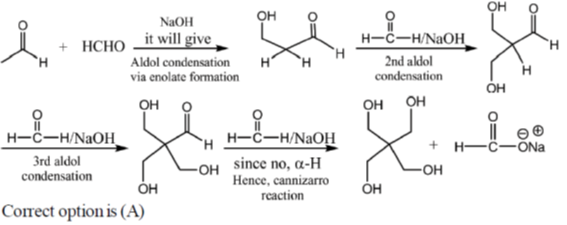
4. The most basic amino acid among the following is
(a) tyrosine
(b) methionine
(c) arginine
(d) glutamine
Ans. c
Sol. If the number of amine (basic) groups are more than carboxylic (acidic) groups then the amino acid will be basic in nature.
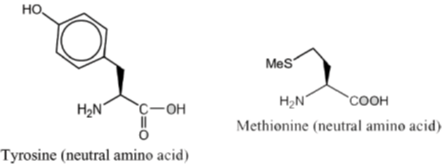
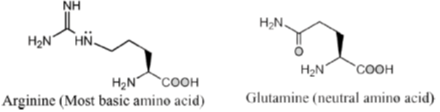
Hence, correct option is (C)
5. The crystal field stabilization energy (CFSE) in [Mn(H2O)6]2+ is
(a) 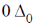
(b) 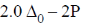
(c) 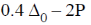
(d) 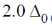
Ans. a
Sol. 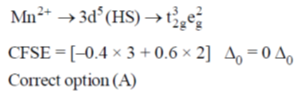
6. Indicator used in redox titration is
(a) Eriochrome black T
(b) Methyl orange
(c) Phenolphthalein
(d) Methylene blue
Ans. d
Sol. Methylene blue is an indicator used in redox titration.
In oxidised form it is blue and will turn colourless if exposed to reducing agent.
Correct option is (D)
7. Among the following, the compound that has the lowest degree of ionic character is
(a) NaCl
(b) MgCl2
(c) AlCl3
(d) CaCl2
Ans. c
Sol. More the polaristion, lesser will be ionic character.

Therefore, polarisation increases (Fajan Rule)
Therefore, co-valent character increases
Therefore, ionic character decreases.
Correct option is (C)
8. The correct order of entropy for various states of CO2 is
(a) CO2(s) > CO2(l) > CO2(g)
(b) CO2(l) > CO2(s) > CO2(g)
(c) CO2(g) > CO2(l) > CO2(s)
(d) CO2(g) > CO2(s) > CO2(l)
Ans. c
Sol. Randomness of the gas is more that of liquid, and randomness of liquid is more than that of solid.
Correct option is (C)
9. The coordination numbers of Cs+ and Cl– ions in the CsCl structure, respectively are
(a) 4, 4
(b) 4, 8
(c) 6, 6
(d) 8, 8
Ans. d
Sol. Cs+ = at body centre
Cl– = At corners
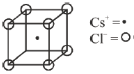
One Cs+ ion surrounded by 8 Cl– ion and vice versa.
Correct option is (D)
10. Determinant of a square matrix is always
(a) a square matrix
(b) a column matrix
(c) a row matrix
(d) a number
Ans. d
Sol. In the case of square matrix, the determinant of the matrix is always gives out a number i.e. 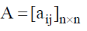 then |A| = B where B is any number.
then |A| = B where B is any number.
Correct option is (D)
11. The correct order of 1HNMR chemical shift  values for the labeled methyl groups in the following compound is
values for the labeled methyl groups in the following compound is
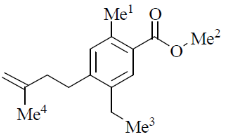
(a) Me1 < Me2 < Me3 < Me4
(b) Me3 < Me4 < Me1 < Me2
(c) Me3 < Me1 < Me4 < Me2
(d) Me2 < Me4 < Me3 < Me1
Ans. b
Sol. –O–Me2 protons deshielded by –I effect of oxygen.
Me3 lies in range of  1-2 ppm i.e. alkyl region, highly shielded. Me3 has lower
1-2 ppm i.e. alkyl region, highly shielded. Me3 has lower  value.
value.
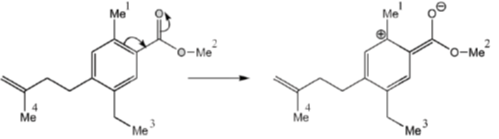
Me1 and Me4 have similar 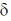 value, but due to –R and –I effect of –COMe2 group, Me1 has slightly more
value, but due to –R and –I effect of –COMe2 group, Me1 has slightly more 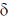 value than Me4.
value than Me4.
Correct order is Me3 < Me4 < Me1 < Me2.
So, correct option is (B)
12. Among the following, the most stable conformation of meso-2,3-dibromobutane is
(a) 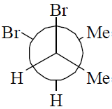
(b) 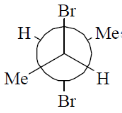
(c) 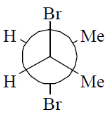
(d) 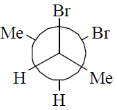
Ans. b
Sol. 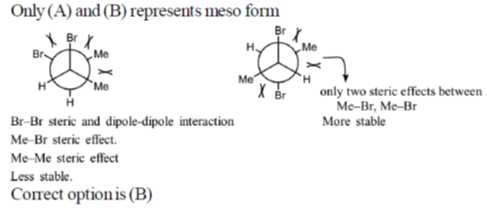
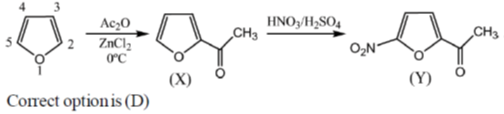
13. The major product X and Y in the following reaction sequence are
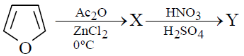
(a) 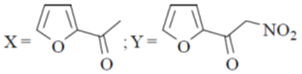
(b) 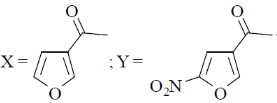
(c) 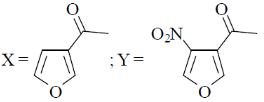
(d) 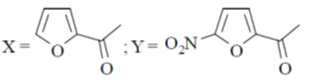
Ans. d
Sol. Electrophilic substitution reactions of Furan occurs at second position and if the second position is occupied then further electrophilic substitution occurs at fifth position, because the positive charge is stabilized by the oxygen as well as  of the ring.
of the ring.
14. The major product formed in the reaction of butanenitrile with phenylmagnesium bromide followed by acdification is
(a) 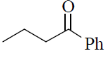
(b) 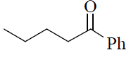
(c) 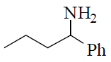
(d) 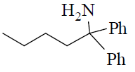
Ans. a
Sol. 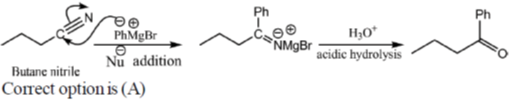
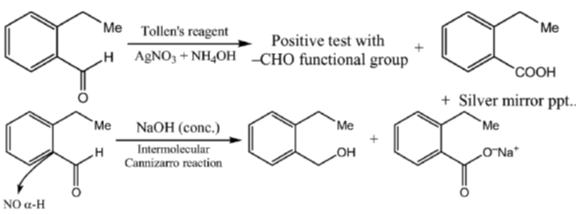
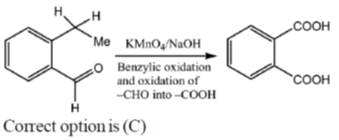
15. An organic compound on reaction with 2,4-dinitrophenylhydrazine (2, 4-DNP) gives a yellow precipitate. It also gives silver mirror on reaction with ammoniacal AgNO3. It gives an alcohol and sodium salt of a carboxylic acid on reaction with concentrated NaOH. It yields benzene-1,2-dicarboxylic acid on heating with alkaline KMnO4. The structure of the compound among the following is
(a) 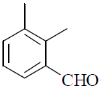
(b) 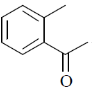
(c) 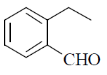
(d) 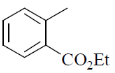
Ans. c
Sol. 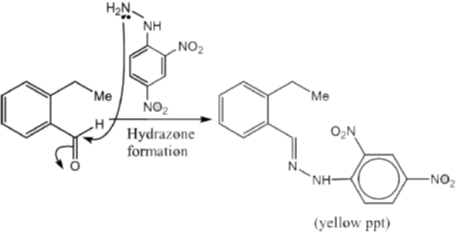
16. The major products X and Y in the following reaction sequence are
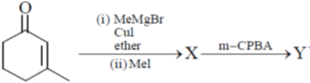
(a) 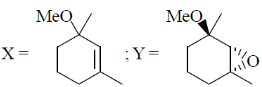
(b) 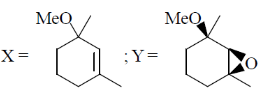
(c) 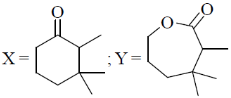
(d) 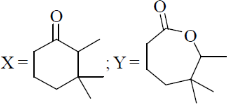
Ans. d
Sol. 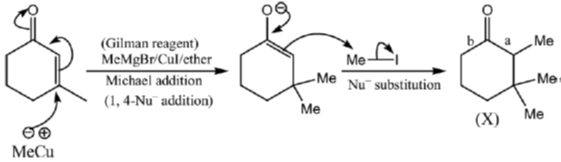
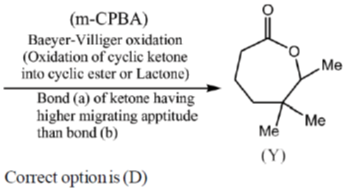
17. The true statement about [Cu(H2O)6]2+ is
(a) All Cu–O bond lengths are equal
(b) One Cu–O bond length is shorter than the remaining five
(c) Three Cu–O bond lengths are shorter than the remaining three
(d) Four Cu–O bond lengths are shorter than the remaining two
Ans. d
Sol. 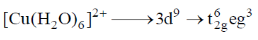
The eg orbitals are unsymmetrically filled. Thus, due to John-Teller distortion four Cu-O bond lengths are shorter than the remaining two.
Correct option is (D)
18. The complexes [Pt(CN)4]2– and [NiCl4]2–, respectively, are
(a) paramagnetic, paramagnetic
(b) diamagnetic, diamagnetic
(c) paramagnetic, diamagnetic
(d) diamagnetic, paramagnetic
Ans. d
Sol. [Pt(CN)4]2– is square planar and diamagnetic, because d8 square planar complexes are diamagnetic.
[NiCl4]2– is tetrahedral and paramagnetic (d8 : e4t24)
Correct option is (D)
19. The value of 'x' in [Cu(CO)x]+ such that it obeys the 18 electron rule is
(a) 6
(b) 5
(c) 4
(d) 3
Ans. c
Sol. Value of 'x' in [Cu(CO)x]+
Valence electron in Cu = 3d104s1
Cu+ = 3d10. So, 2x + 10 = 18

Correct option is (C)
20. The correct order of 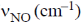 in the following compound is
in the following compound is
(a) NO+ > NO > [NiCp(NO)] > [Cr(Cp)2(NO)4]
(b) [Cr(Cp)2(NO)4] > [NiCp(NO)] > NO+ > NO
(c) NO+ > [Cr(Cp)2(NO)4] > NO > [NiCp(NO)]
(d) [NiCp(NO)] > NO > [Cr(Cp)2(NO)4] > NO+
Ans. a
Sol. 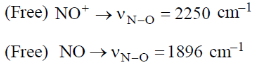
In NO+ bond order is 3 and in NO bond order is 2.5. Higher the bond order, higher the bond strength. So, higher the bond strength higher the frequency, 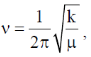 where k is bond strength.
where k is bond strength.

Due to back  the bond order of N-O will decrease and hence N-O stretching frequency decreases.
the bond order of N-O will decrease and hence N-O stretching frequency decreases.
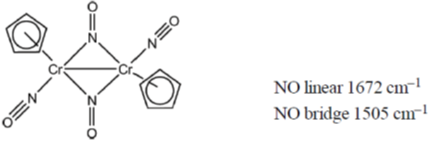
Due to more back  in the complex, the N-O bond order will decreases and its
in the complex, the N-O bond order will decreases and its 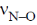 stretching frequency decreases consequently.
stretching frequency decreases consequently.
Correct option is (A)
21. The red colour of ruby is due to
(a) d-d transition of Cr3+ ion in Cr2O3 lattice
(b) d-d transition of Cr3+ ion in Al2O3 lattice
(c) ligand to metal charge transfer transition
(d) metal to metal charge transfer transition
Ans. b
Sol. Due to d-d transition of Cr3+ ion in Al2O3 lattice.
Correct option is (B)
22. The final products in the reaction of BF3 with water are
(a) B(OH)3 and OF2
(b) H3BO3 and HBF4
(c) B2O3 and HBF4
(d) B2H6 and HF
Ans. b
Sol. BF3 is a Lewis acid.
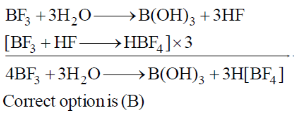
23. The correct order of bond angles in BF3, NH3, NF3 and PH3 is
(a) BF3 > NH3 > NF3 > PH3
(b) PH3 > BF3 > NF3 > NH3
(c) PF3 > PH3 > NH3 > NF3
(d) NH3 > NF3 > BF3 > PH3
Ans. a
Sol. BF3 is trigonal planar hence
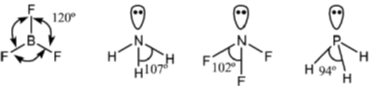
The bond angle decrease, if the electronegativity of the surrounding atom increases (VSEPR theory)
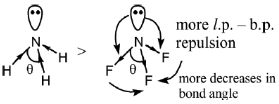
If the electronegativity of central atom decreases, bond angle decreases (VSEPR theory)
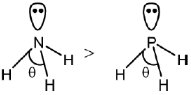
The correct bond order, BF3 > NH3 > NF3 > PH3.
Correct option is (A)
24. The maximum of a function 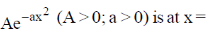
(a) 0
(b) 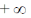
(c) 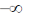
(d) 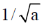
Ans. a
Sol. 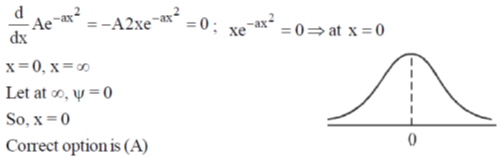
25. At 298 K, 0.1 mol of ammonium acetate and 0.14 mol of acetic acid are dissolved in 1 L of water. The pH of the resulting solution is
[Given: pKa of acetic acid is 4.75]
(a) 4.9
(b) 4.6
(c) 4.3
(d) 2.3
Ans. b
Sol. 0.1 mole of ammonium acetate in 1L of water, 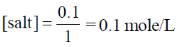
0.14 mole of acetic acid in 1L of water, 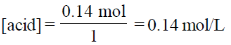
As it is an acidic buffer solution.
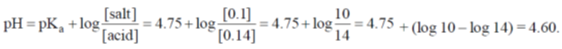
Correct option is (b)
26. An electrochemical cell consists of two half-cell reactions
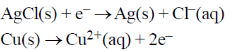
The mass of copper (in grams) dissolved on passing 0.5 A current for 1 hour is
[Given: atomic mass of Cu is 63.6; F = 96500 C mol–1]
(a) 0.88
(b) 1.18
(c) 0.29
(d) 0.59
Ans. d
Sol. Method - 1:
Amount of charge passed = i × t = 0.5 × 60 × 60 coulomb.
Number of Faraday passed 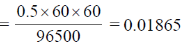
Since, two Faraday disolves 1 mole of Cu.
Therefore, So, 0.01865 Faraday's will dissolve 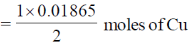
So, weight of Cu dissolved 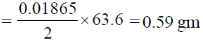

27. For a zero order reaction, the half-life depends on the initial concentration [C0] of the reactant as
(a) [C0]
(b) [C0]0
(c) [C0]–1
(d) [C0]1/2
Ans. a
Sol. 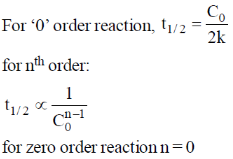
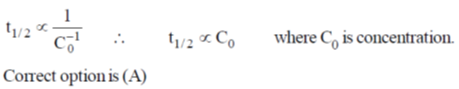
28. The effective nuclear charge of helium atom is 1.7. The first ionization energy of helium atom in eV is
(a) 13.6
(b) 23.1
(c) 39.3
(d) 27.2
Ans. c
Sol. 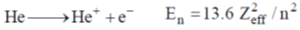
where, Zeff is effective nuclear charge
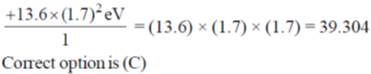
29. The relationship between the van der Waals 'b' coefficient of N2 and O2 is
(a) b(N2) = b(O2) = 0
(b) 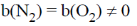
(c) b(N2) > b(O2)
(d) b(N2) < b(O2)
Ans. c
Sol. As 'b' represents (4 × molecular volume per mole)
b(N2) > b(O2) As molecular of N2 is larger than O2.
Correct option is (C)
30. From the kinetic theory of gases, the ratio of most probable speed (Cmp) to root mean square speed (Crms) is
(a) 
(b) 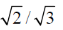
(c) 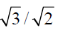
(d) 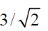
Ans. b
Sol. 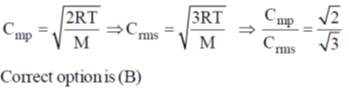
31. The correct statement(s) about the following species is(are)
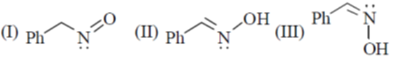
(a) I and II are resonance structures
(b) II and III are resonance structures
(c) II and III are diastereomers
(d) III is a tautomer of I
Ans. c,d
Sol. 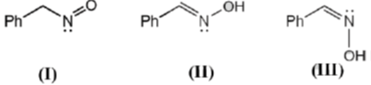
In resonating structure there is no shift of atom takes place.
cis and trans stereoisomer are a pair of diastereoisomers.
Hence, III and II are diastereoisomer and III is also a tautomeric form of I.
Hence (C), (D) is correct.
32. Consider the following reaction:
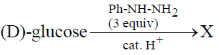
Among the following, the compound(s) whose osazone derivative(s) will have the same melting point as that of X is(are)
(a) 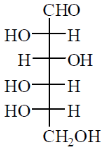
(b) 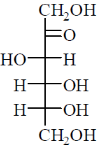
(c) 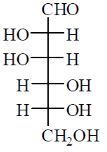
(d) 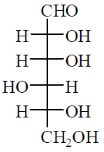
Ans. a,b,c
Sol. All C2 epimers form same osazone moiety. During osazone formation the chirality of C2 carbon is lost.
For example: D-glucose, D-mannose are C2-epimers and they form same osazone. Fructose also form the same osazone like that of D-glucose.
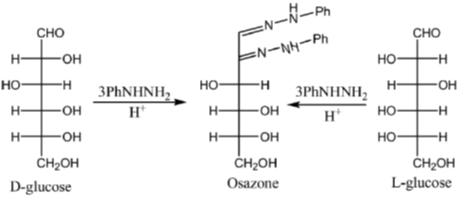
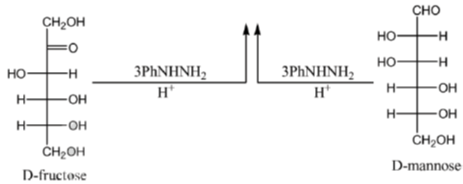
The osazone formed by D-glucose, L-glucose, D-mannose and D-fractose are structurally similar. Hence, they have the same melting point.
Correct options are (A), (B) and (C).
33. The appropriate reagents required for carrying out the following transformation are
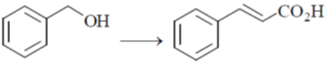
(a) (i) PCC, CH2Cl2; (ii) Ph3P = CHCO2Et; (iii) aq. NaOH, heat, then acidify
(b) (i) CrO3, H2SO4, aq. acetone (ii) Ac2O, NaOAc
(c) (i) MnO2; (ii) CH2(CO2H)2, piperidine, pyridine
(d) (i) PCC, CH2Cl2; (ii) BrCH2CO2C(CH3)3, Zn (iii) H3O+, heat
Ans. a,c,d
Sol. 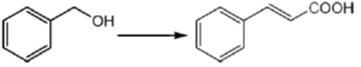
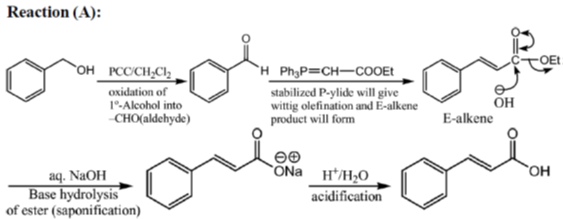
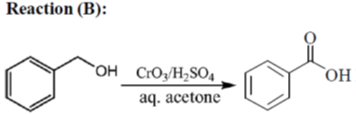
All primary alcohols oxidized with Johe's reagent and give overoxidation product due to formation of stable hydrate formed after aldehyde formation.
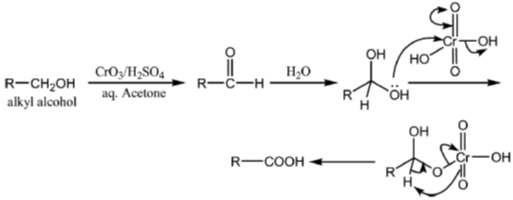
However, Benzyl alcohol and allyl alcohols doesn't form stable hydrates after aldehyde stage oxidation. Hence, can be selectively.
Oxidized to aldehyde.
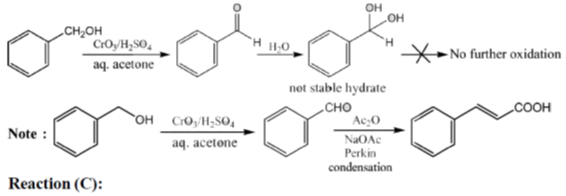
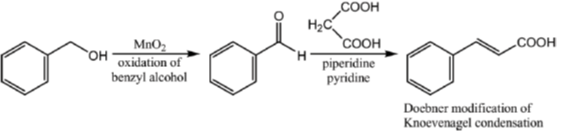
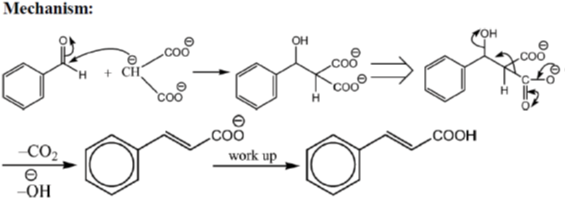
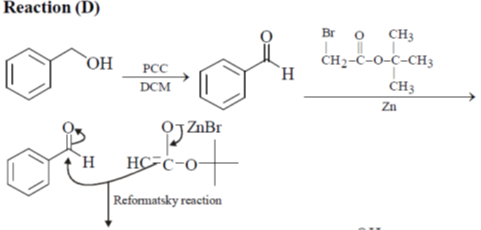
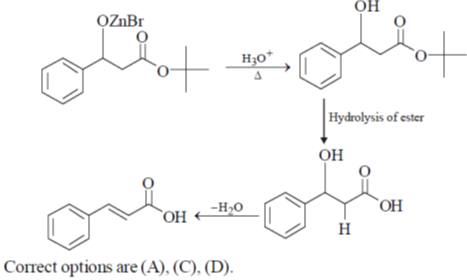
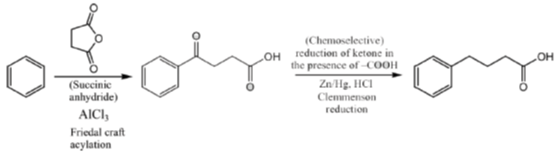
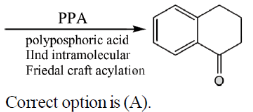
34. The appropriate reagents required for carrying out the following transformation are
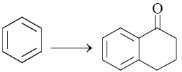
(a) (i) succinic anhydride, AlCl3; (ii) Zn/Hg, HCl; (iii) polyphosphoric acid
(b) (i) maleic anhydride, AlCl3; (ii) H2N-NH2, KOH; (iii) H2SO4
(c) (i) succinic anhydride, FeCl3; (ii) LiAlH4; (iii) H2SO4
(d) (i) Phthalic anhydride, F3B · OEt2; (ii) HS(CH2)2SH, H+; (iii) Raney Ni; (iv) polyphosphoric acid
Ans. a
Sol. 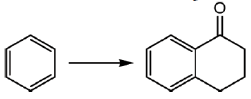
35. The protein(s) that belong to the class of blue copper protein is(are)
(a) ceruloplasmin
(b) superoxide dismutase
(c) hemocyanin
(d) azurin
Ans. a,c,d
Sol. Ceruloplasmin, hemocyanin and azurin are blue copper proteins. Super oxide dismutase may be Cu-ZnSOD, MnSOD or FeSOD.
Correct option are (A), (C) and (D)
36. The ion(s) that exhibit only charge transfer bands in the absorption spectra (UV-visible region) is(are)
(a) [Cr(C2O4)3]3–
(b) [CrO4]2–
(c) [ReO4]–
(d) [NiO2]2–
Ans. b,c
Sol. In CrO42– and ReO4–, Cr+6 and Re+7 have vacant orbital and there is only LMCT.
Correct option are (B) and (C).
37. The type(s) is interaction(s) that hold layers of graphite together is(are)
(a) 
(b) van der Waals
(c) hydrogen bonding
(d) Coulombic
Ans. a,b
Sol. Graphite has van der Waal's forces that holds layers of it. With 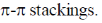
Correct options are (A) and (B).
38. True Statement(s) about Langmuir isotherm is(are)
(a) valid for monolayer coverage
(b) all adsorption sites are equivalent
(c) there is dynamic equilibrium between free gas and adsorbed gas
(d) adsorption probability is independent of occupancy at the neighboring sites
Ans. a,b,c,d
Sol. According to Langmuir adsorption isotherm
• Adsorption is monolayer
• The gasesous molecules adsorbed at different sites do not interact with each other.
• The phenomenon of adsorption involves a dynamic equilibrium between free gas and adsorbed gas.
•Each site can hold only one gaseous molecule and involves a constant heat of adsorption. The latter is identical for all adsorption sites.
Therefore, correct option is (A), (B), (C), (D)
39. The 3pz orbital has
(a) one radial node
(b) two radial nodes
(c) one angular node
(d) two angular nodes
Ans. a,c
Sol. 3pz
n = 3, l = 1
Number of radial node = n – l – 1 = 3 – 1 – 1 = 1
Number of angular node = l = 1
Correct options are (A) and (C).
40. The diatomic molecule(s) that has (have) two 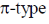 bonds is(are)
bonds is(are)
(a) B2
(b) C2
(c) N2
(d) O2
Ans. b,c
Sol. The configuration are
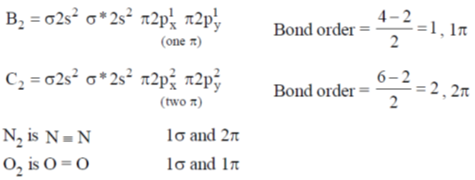
hence, correct options are (B) and (C).
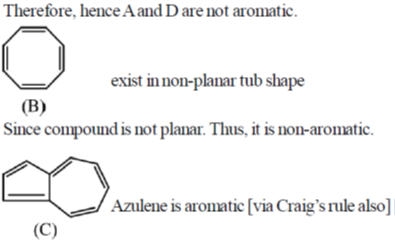
41. Among the following, the number of molecules that are aromatic is ____.
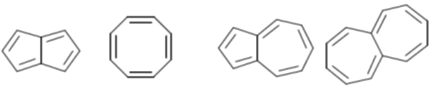
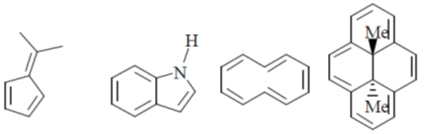
Ans. 3
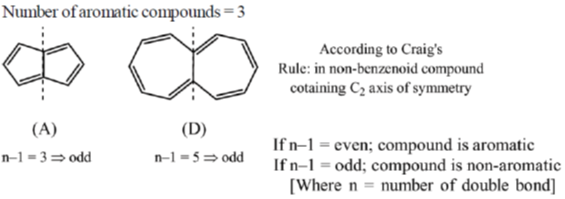
Sol. 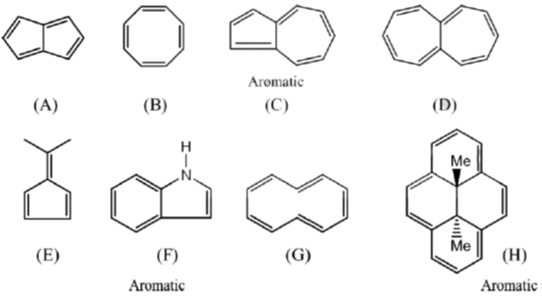

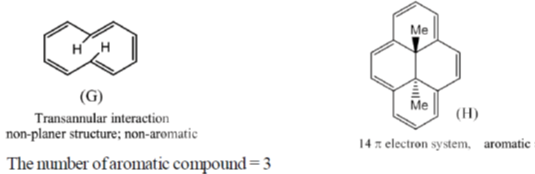
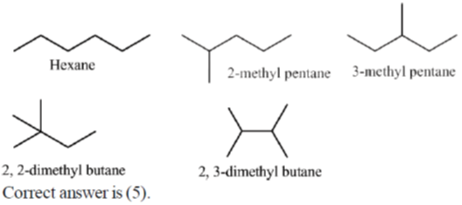
42. The number of all possible isomers for the molecular formula C6H14 is ____.
Ans. 5
Sol. C6H14 hexane
43. Hydrolysis of 15.45 g of benzonitrile produced 10.98 g of benzoic acid. The percentage yield of acid formed is _____.
Ans. 60
Sol. 
Since, idealy 103 gm of C6H5CN forms 122 gm of CH3COOH
Therefore, 15.4 gm of C6H5CN will form 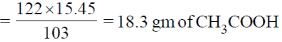
But actual CH3COOH formed = 10.98 gm
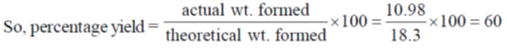
44. Acetic acid content in commercial vinegar was analyzed by titrating against 1.5 M NaOH solution. A 20 mL vinegar sample required 18 mL of titrant to give endpoint. The concentration of acetic acid in the Vinegar (in mol L–1) is ______.
Ans. 135
Sol. At end point
Millimoles of CH3COOH = millimoles of NaOH
M1V1 = M2V2; M1(20) = 1.5(18)
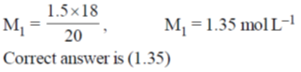
45. The bond order of Be2 molecule is ____.
Ans. 0
Sol. 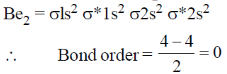
46. The number of P-H bonds in hypophosphorus acid is _____.
Ans. 2
Sol. 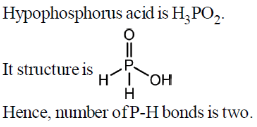
47. The isotope 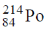 undergoes one alpha and one beta particle emission sequentially to form an isotope "X". The number of neutrons in "X" is _____.
undergoes one alpha and one beta particle emission sequentially to form an isotope "X". The number of neutrons in "X" is _____.
Ans. 127
Sol. 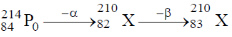
Therefore, number of neutron = 210 – 83 = 127.
48. In a diffrection experiment with X-rays of wavelength 1.54 Å, a diffraction line corresponding to 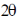 = 20.8º is observed. The inter-planar separation in Å is _____
= 20.8º is observed. The inter-planar separation in Å is _____
Ans. 4.266
Sol. 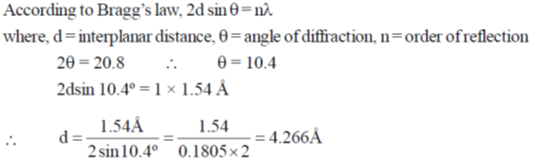
49. The potential energy of interaction between two ions in an ionic compound is given by 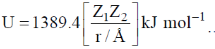 Assuming that CaCl2 is linear molecule of length 5.6 Å, the potential energy for CaCl2 molecule in kJ mol–1 is___.
Assuming that CaCl2 is linear molecule of length 5.6 Å, the potential energy for CaCl2 molecule in kJ mol–1 is___.
Ans. –1736.7
Sol. 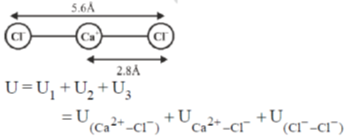

50. The enthalpy of formation for CH4(g), C(g) and H(g) are –75, 717 and 218 kJ mol–1, respectively. The enthalpy of the C – H bond in kJ mol–1 is ____.
Ans. 416
Sol. 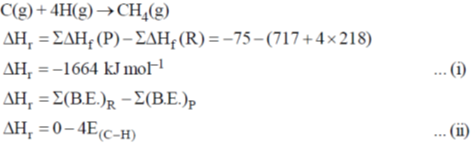
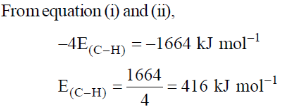
51. Specific rotation of the (R)-enantiomer of a chiral compound is 48. The specific rotation of a sample of this compound which contains 25% of (S)-enantiomer is ____.
Ans. 24º
Sol. Specific rotation of R = 48º
Sample = 25% S with 75% R
R-isomer is in excess. So, specific rotation of mixture will have +ve sign.
Enantiomeric excess (e.e.) = 75% R–25%S = 50%

52. Among the following, the number of compounds, which can participate as 'diene' component in a Diels-Alder reaction is ____.
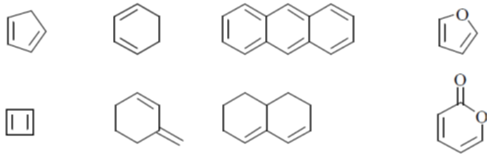
Ans. 6
Sol. Only cisoid form of Dienes undergoes Diels-Alder reaction
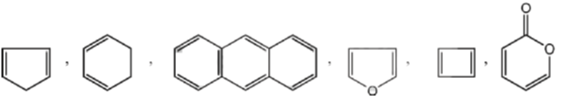
All these compounds are in cisoid form and they given Diels-Alder reaction.
Furan and anthracene are less aromatic and undergoes Diels-Alder reaction.
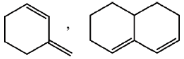
These two Dienes are in transoid form and they will not give the Diels-Alder reaction.
Hence, correct answer is (6).
53. Among the following, the number of molecules that possess C2 axis of symmetry is ____.
Ans. 7
Sol. 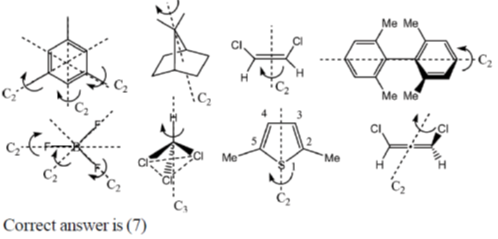
54. Effective nuclear charge for 3d electron in vanadium (atomic number = 23) according to Slater's rule is ____.
Ans. 4.3
Sol. z = 23 = 1s22s22p63s23p64s23d3
or (1s2)(2s2p)8(3s3p)8(3d)3(4s)2
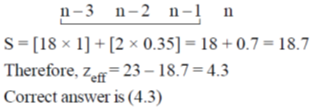
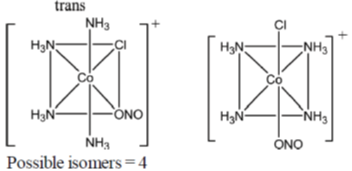
55. The total number of isomers possible for the molecule [Co(NH3)4Cl(NO2)]+ is ____.
Ans. 4
Sol. [Co(NH3)4Cl(NO2)]+


56. The bond angle in PBr3 is 101º. The percent 's' character of the central atom is ____.
Ans. 16.0228
Sol. 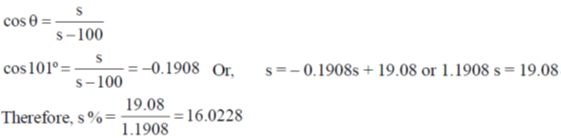
57. 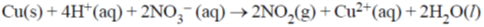
In the above reaction at 1 atm and 298 K, if 6.36 g of copper is used. Assuming ideal gas behavior, the volume of NO2 produced in liters is ____.
[Given: atomic mass of Cu is 63.6; R = 0.0821 L atm K–1 mol–1]
Ans. 4.89
Sol. 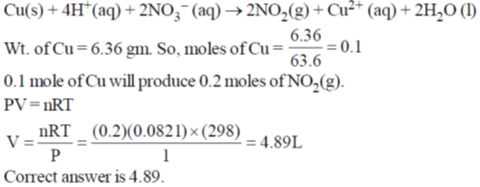
58. The 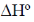 for the reaction CO(g) + ½O2(g) → CO2(g) at 400 K in kJ mol–1 is ____.
for the reaction CO(g) + ½O2(g) → CO2(g) at 400 K in kJ mol–1 is ____.
Given at 298 K:
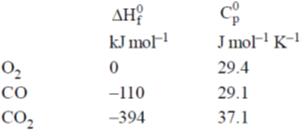
Ans. -284.68
Sol. 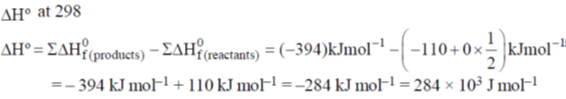
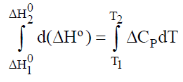
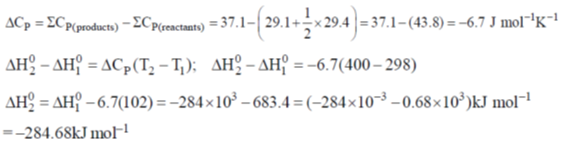
59. The rate constants for a reaction at 300 and 350 K are 8 and 160 L mol–1 s–1, respectively. The activation energy of the reaction in kJ mol–1 is ____.
[Given R = 8.314 J K–1 mol–1]
Ans. 52.30368
Sol. 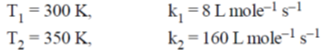
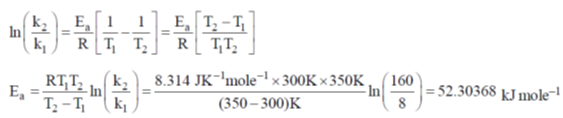
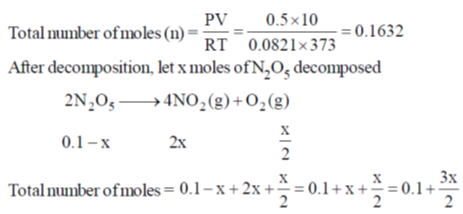
60. A 10 L flask containing 10.8 g of N2O5 is heated to 373 K, which leads to its decomposition according to the equation 2 N2O5(g)  4 NO2(g) + O2(g). If the final pressure in the flask is 0.5 atm, then the partial pressure of O2(g) in atm is ____.
4 NO2(g) + O2(g). If the final pressure in the flask is 0.5 atm, then the partial pressure of O2(g) in atm is ____.
[Given R = 0.0821 L atm K–1 mol–1]
Ans. 0.0643
Sol.