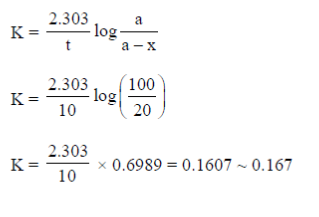IIT JAM Biology 2020
Previous Year Question Paper with Solution.
1. Deficiency of the enzyme phenylalanine hydroxylase causes phenylketonuria. Phenylalanine hydroxylase converts phenylalaine to
(a) tryptophan
(b) alanine
(c) tyrosine
(d) threonine
Ans. (c)
Sol. Phenylketonuria (PKU) is an autosomal recessive disorder of metabolism caused by a deficiency in the liver enzyme phenylalanine hydroxylase. Which is responsible for the conversion of phenylalanine to another amino acid tyrosine. In 19347, A Folling discovered an autosomal recessive mutation of gene on chromosome 12, called phenylketonuria. This abnormal recessive gene causes phenylalanine to build utp potentially toxic level in the body.
The affected babies are normal at birth, but within a few weeks, there is rise in plasma phenylalanine level which inhibits the development of brain. Usually by six months of life, severe mental retardation is evident.
2. T-cells and B-cells are
(a) lymphocytes
(b) erythrocytes
(c) epithelial cells
(d) squamous cells
Ans. (a)
Sol. B-cells and T-cells are the two types of lymphocytes that are involved in triggering the immune response in the body. T-cells (thymus cells) are involved in cell-mediated immunity and B-cells (bone narrow or bursa-derived cells) are primarily responsible for humoral immunity.
3. In humans, the testis temperature in maintained below the care body temperature with the help of
(a) proximal tubule
(b) loop of Henle
(c) scrotum
(d) serminal vesicles
Ans. (c)
Sol. In human males, the testes are situated outside the abdominal or pelvic cavity in a sec of skin called the scrotal sac. It acts as a thermoregulator to keep the testicular temperature 2-3°C lower than the normal internal body temperature. This is the optimum temperature necessary for sperm production (spermatogenesis). The scrotum remains connected with the abdomen or pelvic cavity by the inguinal canals.
4. Protein that helps other proteins to fold correctly is
(a) chaperone
(b) proteasome
(c) ubiquitin
(d) desmosome
Ans. (a)
Sol. Chaperones are a group of proteins that have functional similarity and assist in protein folding. They are protein that have the ability to prevent non-specific aggregation by binding to non-native proteins. Example of chaperon proteins is the Heat shock proteins (Hsps).
5. The correct sequence of phases during mitosis is
(a) prophase, metaphase, anaphase, telophase
(b) prophase, anaphase, metaphase, telophase
(c) anaphase, prophase, metaphase, telophase
(d) anaphase, metaphase, prophase, telophase
Ans. (a)
Sol. Mitosis consists of four basic phases, i.e., prophase, metaphase, anaphase and telophase, resptectively and followed by cytokinesis, the process of dividing the cell contents i.e., cytoplasm, to make two new cells.
⚫ During prophase the chromatin fibers become shorten and thicken. Each chromosome consist of two chromatids, Nucleoli and nuclear envelope is no longer visible.
⚫ During metaphase the chromosomes lines up at the equator of the spindle to form metaphse plate. The kinetochores of sister chromatids get joined to spindle fibres from opposite poles.
⚫ During anaphase the sister chromatids of each chromosome starts moving to the opposite poles of the spindle. Splitting of chromosomes takes place due to the division of centromere which attaches two chromatids.
⚫ During telophase nuclear envelope is reformed around the chromatids, nucleolus reforms in each nucleus, chromosomes decondense into long line filaments and mitotic spindle disappears.
6. The curve y = x4 – 4x3 + 4x2 – 4 has tangents parallel to the x-axis at the following ponts (x, y)
(a) (1, 4), (–2, 2) and (0, –1)
(b) (0, –4), (2, – 4) and (1, –3)
(c) (–1, 2), (–2, 1) and (1, –2)
(d) (1, –4), (1, –3) and (2, –4)
Ans. (b)
Sol. y = x4 – 4x3 + 4x2 – 4

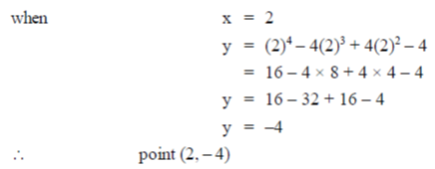
7. In viruses, capsids are made up of
(a) proteins
(b) nucleic acids
(c) lipids
(d) sterols
Ans. (a)
Sol. Capsid is the outer protective coat of a virus, which is mostly made up of specific proteins. The identical subunits of capsid are called capsomeres. The shape and arrangement of capsomeres determines the shape or symmetry of a virus. The capsid protects the underlying nucleic acid. Besides protection, it also serves as a vehicle of transmission for viral nucleic acid from one host to another.
8. The common component in crustacean exoskeleton and fungal cell wall is
(a) lignin
(b) cellulose
(c) chitin
(d) peptidoglycan
Ans. (c)
Sol. Chitin is the principal structure component of exoskeleton of invertebrates, e.g. arthrpods (Crustanceans) and is also a major cell wall contituent of most fungi and many algae. It is second most abundant carbohydrate and a homopolymer of N-acetyl glucosamine (NAG) joined with  -1, 4-linkage.
-1, 4-linkage.
9. The correct sequence of evolution (simplest to complex) is
(a) algae, bryophytes, ferns, angiosperms
(b) algae, ferns, bryophytes, angiosperms
(c) bryophytes, ferns, algae, angiosperms
(d) bryophytes, algae, ferns, angiosperms
Ans. (a)
Sol. The correct sequence of evolution (simplest to complete) is algae, bryophytes, ferns and angiosperms.
The evolution of plants has resulted in wide range of complexity, through multicelluar marine and freshwater green alage, terrestrial bryophytes, lycopods and ferns to the complex gymnosperms and angiosperms.
10. For an ideal gas at room temperature, choose the correct representation(s) of Boyle's Law. (p = Pressure, V = Volume, T= Temperature)
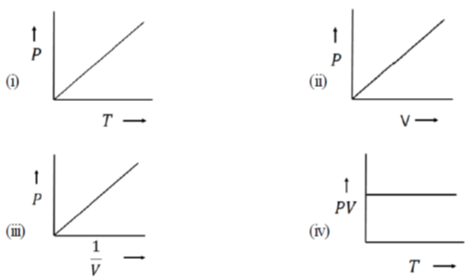
(a) Only (i)
(b) Both (ii) and (iii)
(c) Only (iii)
(d) Both (iii) and (iv)
Ans. (c)
Sol. Boyle's law states that for a given mass of a gas at constant temperature, the volume of that mass of gas is inversely proportional to its pressure
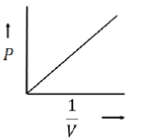
Mathematically, Boyle's law can be stated as
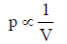
So, the graph given in (iii) is correct.
11. During El Nino
(a) cold water of the north flowing Peru current is displaced by a low-nutrient warm southward current
(b) warm water of the north flowing Peru current is displaced by a low-nutrient cold southward current
(c) cold water of the south flowing peru current is displaced by a warm northward current rich in nutrietns
(d) warm water of the south flowing peru current is displaced by a cold northward currect rich in nutrients
Ans. (a)
Sol. During El nino, acid water of the North flowing Perv current is displaced by a low nutrient warm southward current. The surface water in the central and eastern pacific ocean become significant warmer than usual during El nino phenomenon.
It also reduces the upwelling of cooler, nutrient rich waters from the deep shutting down or reversing ocean currents along the equator and the west coast of South and central America.
12. Match the deficiency conditions in Group I with the corresponding vitamin in Group II.

(a) P-3, Q-2, R-1, S-4
(b) P-2, Q-3, R-4, S-1
(c) P-3, Q-1, R-4, S-2
(d) P-1, Q-2, R-3, S-4
Ans. (c)
Sol. 1.Vitamin-B1 is called as thiamine. The deficiency of this vitamin results in beri-beri disease. If affects the nervous and the circulatory system.
2.Vitamin-C is called as ascorbic acid. The deficiency of this vitamin causes scurvy disease. Scuvy is characterised by bleeding gums, rash, fatigue and weak immunity.
3.Vitamin-B9 is called as folates or follic acid. Pregnant women who do not get enough folic acid are more likely to have children with birth defects.
4.Viramin-A is also called as retinol. The deficiency of this vitamin results in loss of eyesight or night blindness. Nyctalopia is the term used to refer night blindness.
13. Eutrophication refers to an aging process from a
(a) low production ecosystem to high production ecosystem due to availability of excess nutrients
(b) high production ecosystem to low production ecosystem due to nutrient deficiency
(c) high production ecosystem to low production ecosystem due to light scarcity
(d) low production ecosystem to high production ecosystem due to light scarcity
Ans. (a)
Sol. Eutrophication refers to an aging process of a water body from a low production ecosystem to high production ecosystem to high production ecosystem due to availability to excess nutrients. Eutrophication most commonly arises from the oversupply of nutrients, like nitrogen and phosphorus, that cause structural changes to the ecosystem such as increased production of algae and aquatic plants, depletion of dissolve oxygen, general deterioration of water quality and other effects that affect aquatic life and reduce its use.
14. Which one of the following ions has the maximum number of unparied electrons?
(a) Cu2+
(b) Na+
(c) Cr3+
(d) Cr3+
Ans. (d)
Sol. Cu2+ has 1 unparied electron
Na+ has 0 unpaired electron
Cr3+ has 3 unparied electrons and
Fe3+ has 5 maximum unpaired electrons
Or
Cu(Z = 29) = 1s2 2s2 2p6 3s2 3p6 3d10 4s1
Cu2+ = 1s2 2s2 2p6 3s 23p6 3d9
= ione unpaired electron
Na (Z = 11) = 1s2 2s2 2p6 3s1
Na+ = 1s2 2s2 2p6
= 0 unpaired electron
Cr (Z = 24) = 1s2 2s2 2p 63s2 3p6 3d5 4s1
Cr3+ = 1s2 2s2 2p6 3s2 3p6 3d3
= 3 unpaired electron
Fe(Z = 26) = 1s2 2s2 2p 63s2 3p6 3d5 4s1
Fe3+ = 1s2 2s2 2p6 3s2 3p63d5
= 5 unparied electrons
15. Match the enzymes in Group I with the corresponding substrate in Group II.
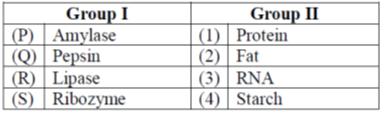
(a) P-2, Q-3, R-1, S-4
(b) P-4, Q-1, R-2, S-3
(c) P-3, Q-1, R-4, S-2
(d) P-4, Q-2, R-3, S-1
Ans. (b)
Sol. Salivary amylase digests into maltose in the buccal cavity.
Pepsin digests protein into its monomers amino acids in the stomach
Pancreatic lipase digests emulsified fat in the small intenstine. Lipase digests fats into the fatty acids and glycerol.
Ribozymes are RNA molecules that catalyse the cleavage of RNA substrates and the formation of covalent bonds in RNA strands at specific sites.
16. In ABO blood group testing, which one of the following is incorrect?
(a) A group-Agglutination with anti-A antibodies
(b) B group-Agglutination with anti-B antibodies
(c) AB group-No agglutination with either anti-A or anti-B antibodies
(d) O group-No agglutination with either anti-A or anti-B antibodies
Ans. (c)
Sol. AB blood group shows agglutination with both anti-A and anti-B antibodies, as it contains both angigens-A and B and lacks antibodies. A, B and O blood groups were discovered first time by Karl Landsteiner (1990) in human beings. AB blood group was reported by de Castello and Steini (1902). Landsteiner confirmed the presence of two types of proteins in blood responsible for their grouping such as antigen on RBC and antibody in plasma.
Both antigen 'A' and antibody 'a' (also called Anti-A) are incompatiable, and antigen 'B' an antibody 'b' (also called Anti-B) are incompatible to each other and cause self-clumping. So these show agglutination when incompatible bloods come together. Thus, AB-blood group is called universal recipient, as it has no antibody in their blood-plasma so can receive blood from the blood group.
(a)Blood group A has A-antigen and b-antibody
(b)Blood group B has B-antigen and a-antibody
(c)Blood group AB has both A and B antigens, but no antibody
(d)Blood group O has a antigen but with both antibodies.
17. If a coin is tossed three, what is the probability that no two successive tosses show the same face?
(a) 0.25
(b) 0.33
(c) 0.20
(d) 0.125
Ans. (a)
Sol. There are 2 × 3 = 8 possible outcomes after lossing a fair coin fairly 3 times. The 8 possible outcomes are TTT, HTT, THT, TTH, HHT, HTH, THH, HHH.
Exactly 2 of 8 possible outcomes results in 3 of the same faces showing up. They are TTT and HHH. Each has a probability of 1/8 of happening and since either showing up satisfactor addresses the criteria for success, the probability as requested
= 2*(1/8) = 1/4 = 0.25 = 25%
18. Match the RNAs in group I with their corresponding function in Group II.
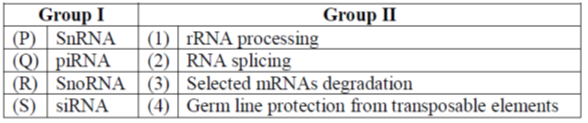
(a) P-4, Q-3, R-2, S-1
(b) P-4, Q-1, R-3, S-2
(c) P-3, Q-2, R-1, S-4
(d) P-2, Q-4, R-1, S-3
Ans. (d)
Sol. 1.A small nuclear RNA (SnRNA) is one of many small RNA species confined to the nucleus, several of the SnRNAs are involved in splicing or other RNA processing reactions.
2.Piwi-interacting RNA (pi RNA) is the largest class of small non-coding RNA molecules expressed in animal cells. The piRNA pathway protects the germline genome against transposable elements.
3.Small nucleolar RNAs (SnoRNAs) are a class of small RNA molecules that primarily guide chemical modifications of other RNAs, mainly ribosomal RNAs, transfer RNAs and small nuclear RNAs. SnoRNAs are generated after splicing, debranching and trimming of mRNA intron.
Mature SnoRNAs associate with proteins to form Small nucleolar ribonucleoproteins (SnoRNPs). These complexes are exported into nucleolus to participate in rRNA processing.
4. Small interfering RNA (SiRNA), is a class of double-stranded RNA, non-coding RNA molecule, 20-25 base paris in length that the generated from cytoplasmic long double-stranded RNA (dsRNA). This cuase selected mRNA degradatiion in a sequence specific manner.
19. In a typical mammalian cell, the protein content is 20% of its net weight. If the density and volume of the cell are 1.2 g/mL and 4 × 10–9 mL, respectively, then the concentration (in mg/mL) of the protein is
(a) 60
(b) 600
(c) 166
(d) 240
Ans. (d)
Sol. Given a typical mammalian cell, the protein content is 20% when the density and volume of the cell are 1.2 g/mL and 4 × 10–9 mL, respectively.
Then, the concentration of the protein
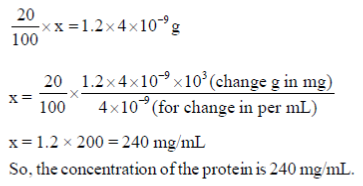
20. Consider a spherial particle of mass m and radius r moving in a medium. Its velocity at any time t is given by v = v0 exp 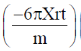 , where v0 is initial velocity of the particle. The dimensions of X are
, where v0 is initial velocity of the particle. The dimensions of X are
(a) MLT–1
(b) M–1LT
(c) ML–1T–1
(d) dimensionless
Ans. (c)
Sol. Velocity of particle is given as
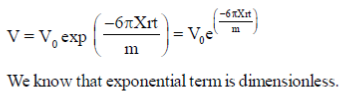
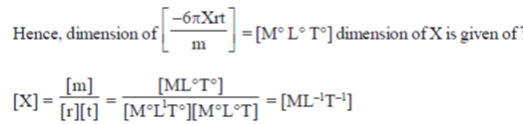
21. Match the type of bacterial flagella in Group I with their definitions in Group II.

(a) P-4, Q-1, R-2, S-3
(b) P-4, Q-3, R-2, S-1
(c) P-4, Q-3, R-1, S-2
(d) P-3, Q-1, R-4, S-2
Ans. (b)
Sol. Depending upon the arrangement of flagella, bacterial cell can be of following types
1.Monotrichous, i.e, with single polar flagellum, e.g. Vibrio cholerae, Thiobacillus and Xanthomonas oryzae.
2.Peritrichous, i.e. flagella distributed over the entire cell, e.g. Escherichia coli, Clostridium and Salmonella.
3.Lophotrichous i.e., with two or more flagella at one end of the cell, e.g. Spirillum serpens, Pseudomonas.
4.Amphitrichous i.e., with single flagellum at each end of the cell, e.g. Nitrosomonas.
22. Consider monatomic ideal gas molecules of equal mass m in thermal equilibrium, at temperature T. Which one of the following equations is correct? (the angular brackets denote average, kB is Boltzmann constant, v is velocity and Vx is the x-component of velocity)
(a) 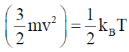
(b) 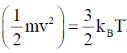
(c) 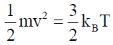
(d) 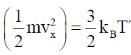
Ans. (b)
Sol. Pressure is described by pV = 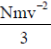 , under ideal gas law pV = NkBT
, under ideal gas law pV = NkBT
Where KB is the Boltzmann constant and T the absolute temperature defined by the ideal gas law, to obtain
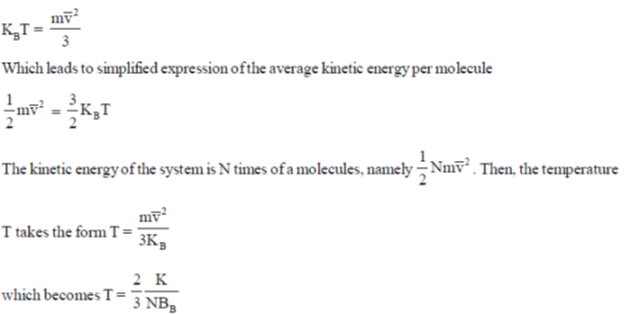
23. Match the diseases in Group I with their causative organisms in Group II.
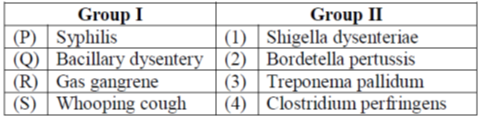
(a) P-2, Q-1, R-4, S-3
(b) P-3, Q-4, R-2, S-1
(c) P-2, Q-3, R-4, S-1
(d) P-3, Q-1, R-4, S-2
Ans. (d)
Sol. 1.Syphilis is a sexually transmitted disease (STD) caused by an infection with bacteria known as Treponema pallidum
2.Bacterial dysentery is an intestinal infection caused by a group of Shigella bacteria such as Shigella dysenteriae which can be found in the human gut. Shigella is a bacillus (rod shape) bacteria, thus this disease is also known as bacillary dysentary.
3.Gas gangrene is caused by infection with the bacterium Clostridium perfringens, which develops in an injury or surgical wound that is depleted of blood supply. The bacterial infection produces toxins that release gas, hence called as 'gas' gangrene and cause tissue death.
4.Pertussis, a respiratory illness commonly known as whooping cough, a very contagious disease caused by a type of bacteria called Bordetella pertussis.
24. Determine the correctness or otherwise of the following Assertion [A] and the Reasion [R]
Assertion [A] The difference in the respective melting points of butter and coconut oil is caused by the degrees of saturation of the corresponding fatty acid chain.
Reason [R] Unsaturated fatty acid chains in fats/lipids become solid more easily because they are relatively straight and thus able to pack together more closely than saturated chain.
(a) Both [A] and [R] are true and [R] is the correct reason for [A]
(b) Both [A] and [R] are true but [R] is not the correct reason for [A]
(c) Both [A] and [R] are false
(d) [A] is true, but [R] is false
Ans. (d)
Sol. The difference in the respective melting piont of bhutter and coconut oil is caused by the degree of saturation of the corresponding fatty acid chain.]
A fatty acid, which have more hydrogen atoms will be more saturated and its melting point will be higher.
The linear molecules are able to come close to each other and create a dense structure, which allows for strong intermolecular interaction.
The melting point of such a fat would be high. An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain.
Therefore, [A] is true but, [R] is false.
25. Match the techniques in Group I with their applications in Group II for protein analysis
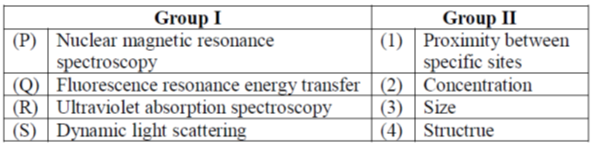
(a) P-3, Q-1, R-2, S-4
(b) P-4, Q-1, R-2, S-3
(c) P-2, Q-1, R-4, S-3
(d) P-3, Q-4, R-1, S-2
Ans. (b)
Sol. 1.Nuclear Magnetic Resonance spectroscopy of protein (protein NMR) is field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins, and also nucleic acids and their complexes.
2.Fluorescence Resonance Energy Transfer (FRET) is a powerful technique that permits as assessment of the distance between two fluorophores, and has been widely applied to assess protein-protein interactions in the cellular setting.
3.UV absorption spectrophotometry can determine the quantity of proteins in the sample by using the maximum absorption at 280 nm. UV absorbance at 280 nm is routinely used to estimate protein concentration in laboratories due to its simplicity, ease of use and affordability.
4.Dynamic light scattering (DLS) is a technique in physics that can be used to determine the size distribution profile of small particles in suspension of polymers in solution.
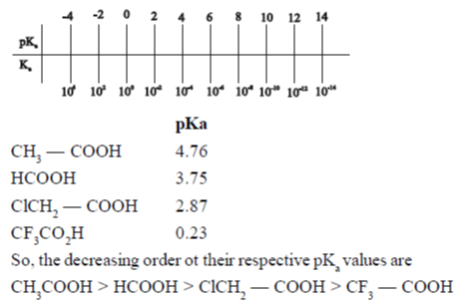
26. The following carboxylic acids have a general formula, R-COOH.
(i)HCOOH(b)CH3-COOH(c)CICH2 - COOH(d)CF3 - COOH
Which one of the following represents the decreasing order of their respective pKa values?
(a) i > ii > iii > iv
(b) ii > i > iii > iv
(c) iv > iii > i > ii
(d) iv > iii > ii > i
Ans. (b)
Sol. The pKa value is the negative logarithm of the acid dissociation constant (Ka) of a solution
pKa = – log10 Ka
The smaller the value of the pKa, the stronger the acid.
27. Which one of the following compounds is the simplest alkane that is optically active?
(a) 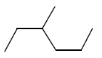
(b) 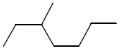
(c) 
(d) 
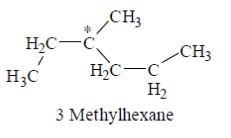
Ans. (a)
Sol. Among the given compound a, b and c have chiral carbon atom so these are optically active. but 3-methyl hexane is the simplest optically active alkane among he given alkane, that is optically active.
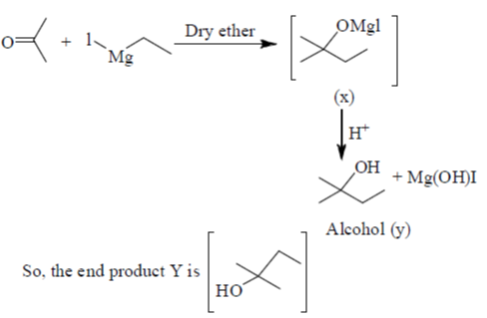
28. In the following reaction, X is an intermediate and Y is one of the end products.
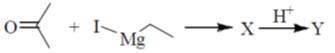
Which one of the following compounds is the end product?
(a) 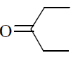
(b) 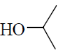
(c) 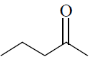
(d) 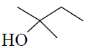
Ans. (d)
Sol.
29. Determine the correctness of otherwise of the following Assertion [A] and the Reason [R]
Assertion [A] lac operon is an inducible operon
Reason [R] lac operon is not induced when the repressor protein remains bound to operator DNA sequence.
(a) Both [A] and [R] are true and [R] is the correct reason for [A]
(b) Both [A] and [R] are true, but [R] is not the correct reason for [A]
(c) Both [A] and [R] are false
(d) [A] is true, but [R] is false
Ans. (a)
Sol. The lac operon is considered as inducible operon because it is usually turned off (repressed), but can be turned on in the presence of the inducer allolactose. With repressible system, the binding of the effector molecule to the repressor greatly increases the affinity of repressor for the operator and the repressor binds and stops transcription.
In the absence of lactose, the repressor remains bound to the operator and preventing access the RNA polymerase to the promoter. Transcription is blocked and the operon is repressed.
In the presence of lactose, the repressor is inactivated form and does not bind to the operator.
Therefore, Assertion (A) and Reason (R) are true and (R) is the correct Reason for (A).
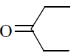
30. IR spectrum of a compound C5H10O shows a band at 1715 cm–1. The same compound showed two signals, a triplet and a quartet, in its NMR spectrum. Identify the compound from the following.
(a) 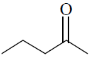
(b) 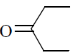
(c) 
(d)
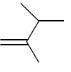
Ans. (b)
Sol. IR spectrem of a compound C5H10O shows a band at 1715cm–1.
The compound is ketone and structure of ketone is
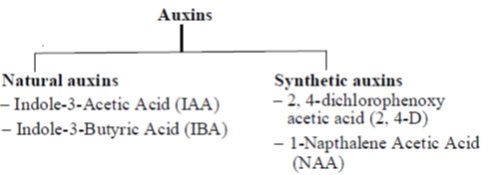
31. Which of the following is(are) auxins?
(a) 1-Naphthaleneacetic acid
(b) Indole-3-butyric acid
(c) 2, 4-Dichlorophenoxyacetic acid
(d) Indole-3-acetic acid
Ans. (a, b, c, d)
Sol. Auxin, was the first plant growth regulator to be discovered. Chemically, auxins are indole derivatives. They are organic acids. These are produced in the growing regions, i.e., stem and root apices. From here, they migrate to their sites of action. Auxins are synthesised from their precursor tryptophan (amino acid).
Auxins are categorised into two main types
32. Which of the following is(are) true about photosynthesis?
(a) In C3-plants, the first organic product of carbon fixation is 3-phosphoglycerate
(b) In C4-plants, the first organic product of carbon fixation is oxaloacetate
(c) Crassulacean acid metabolism occurs in succulent plants living in arid conditions
(d) Oxygen is generated from carbon dioxide.
Ans. (a, b, c)
Sol. In C3 pathway, the first, stable product is 3-phosphoglycerate, whereas in C4 pathway, the first stable product is oxaloacetic acid. C3 pathway occurs in the mesophyll cells of the leaves, whereas C4 pathway occurs in the mesophyll and bundle sheath cells of the leaves. Succulent plants that are adapted to dry environment, such as cacti and pineapple, use the Crassulacean Acid Metabholism (CAM) pathway to minimise photorespiration. The oxgen during photosynthesis comes from split water molecule. The oxygen is just a waste product when plants prform phnotosynthesis.
33. Which of the following is(are) involved in the activation of cytotoxic T-cells?
(a) MHC I
(b) 2, 2 and 1
(c) T cell receptor
(d) CTLA 4
Ans. (a, c)
Sol. MHCl and T-cell receptor are involved in the activation of cytotoxic T-cells. Most cytotoxic T-cells express T-cell Receptros (TCRs) that can recognise a specific antigen.
Antigen inside a cell are bound to class l MHC moelcules and brought to the surface of the cell by the class l MHC molecule, where they can be recognised by the T-cell.
If the TCR is specific for that antigen, it binds to the complex of the class l MHC molecules and the antigen, and the T-cell destroys the cell.
34. DNA and RNA are acidic in nature due to the presence of
(a) pentose sugar
(b) nitrogenous bases
(c) phosphate groups
(d) large number of hydrogen bonds
Ans. (c)
Sol. DNA and RNA are acidic in nature due to the presence of phosphate groups. DNA or RNA are called nucleic acids because of the acidic nature of the phosphate group attached to them.
The phosphodiester bond can easily lose the proton in the presence of nucleophile group subsequently masking the basic nature of nitrogenous bases.
35. Which of the following is(are) correct?
(a) Light has wave nature only
(b) Light can have both wave and particle nature
(c) Photo electric effect shows that light can behave like particles
(d) Interference experiments show that light behaves like particles
Ans. (b, c)
Sol. Quantum mechanisms tell us that light can behave simultaneously as a particle or a wave. Most commonly observed phenomena with light can be explained by waves. But the photoelectric effect suggested a particle nature for light. Experiments show that while a photon was detected as having the properties of a particle interference appeared like that of a wave.
36. Protozoa are
(a) unicellular
(b) multicellular
(c) eukaryotic
(d) prokaryotic
Ans. (a, c)
Sol. The protozoa are a diverse group of unicellular eukaryotic organisms. Protozoa, a traditionally defined are mainly microscopic organisms, ranging in size from 10 to 55 micrometers. However, some are significantly larger such as the deep sea dwelling xenophyophores, single-celled Foraminifera, whose shells can reach 20 cm in diameter.
37. Which of the following curve/straight line equations will pass through the origin when plotted on a graph?
(a) 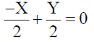
(b) 1 + y + x = 1
(c) xy = 1
(d) 2y – 2x + 2 = 0
Ans. (a, b)
Sol.
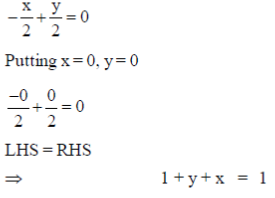
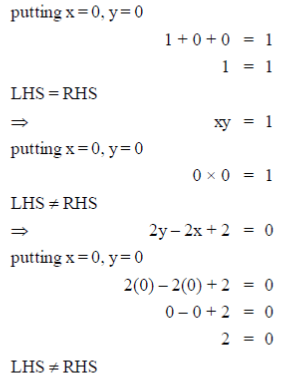
As, the equation (a) and (b) satisfy the condition, i.e. curve/straight line equation will pass through the origin when plotted on a graph.
38. Consider two bodies with equal masses of 1012 kg each and R distance apart. Let G be the gravitational constant and V0 be a constant with dimensions of energy. Which of the following represents(s) gravitational potential energy (V) between the bodies, such that Newton's law of gravitation is valid?
(a) 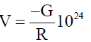
(b) 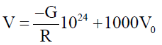
(c) 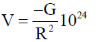
(d) 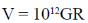
Ans. (a)
Sol. Gravitational potential energy is given by
V(r)2=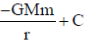
Where,
G = gravitational constant
M = mass of first particle
m = mass of second particle
r = distnace between particles
After placing the value
= 
Where, C = any constant with energy dimension.
39. Which of the following is(are) correct?
(a) Both glucose and fructose have the same molecular formula
(b) The positions of the oxygen and carbon differ in the structures of glucose and fructose
(c) Both glucose and fructose have the same physical properties
(d) Both glucose and fructose are monosaccharides
Ans. (a, b, d)
Sol. Three common sugar. glucose galactose and fructose share the same molecular formula : C6H12O6. But glucose has a six member ring and fructose has a five member ring structure. The position of the oxygen and carbon differ in the structure of glucose and fructose. The anomeric carbon is the first carbon in glucose and second in the fructose. Fructose and glucose are both monosaccharides with the same chemical composition but have different physical properties and a different molecular chemical composition but have different phsical properties and a different molecular structure.
40. Which of the following gas(es) function(s) as signaling molecule(s) in the human nervous system?
(a) Nitric oxide
(b) Carbon monoxide
(c) Helium
(d) Argon
Ans. (a, b)
Sol. Gaseous signaling molecules are gaseous molecules that are either synthesised internall in the organism, tissue or cell or are received by the organism, tissue or cell from outside and that are used to transmit chemical signals which induce certain physiological or biochemical changes in the organism, tissue or cell. The term is applied to, for example oxygen, carbon dioxide, nitric oxide, carbon monoxide, hydrogen sulphide, Sulphur dioxide, nitrous oxide hydrogen cyanide, ammonia, methane, hydrogen and ethylene etc.
Nitric oxide is an autocrine and paracrine signaling molecule that can diffuse the biological signaling molecule that can diffuse the biological membranes. It has a very short duration of action (a few seconds) and its main physiological function is to contribute to the homeostasis of the vasculature.
Carbon monoxide is produced naturally by the human body as signaling molecule. Thus, carbon monoxide may have a physiological role in the body, such as neurotransmitter or a blood vessel relaxant.
41. The generation time of E. coli is 20 minutes. If there are 106 E. coli present in an exponentially growing synchronous culture, then the average time (in minutes) required to obtain a final population of 4 × 106 E.coli is ...............................
Ans. (40)
Sol. Nn = N02n
Nn = no. of cells at any generation
N0 = initial no. of cells
n = number of generation
4 × 106 = 106 × 2n
(2)2 = 2n
n = 2
As, one generation time of E. coli is = 20 min
Two generations will take 20 × 20 = 40 min.
42. The solution to the integral 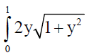 dy, rounded off to two decimal places is ...................
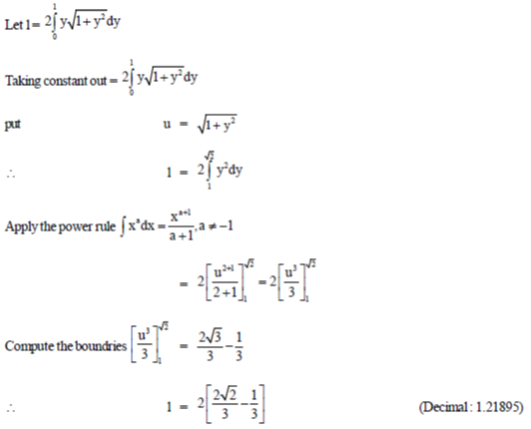
dy, rounded off to two decimal places is ...................
Ans. (1.21)
Sol.
43. Let A = 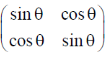 and A + AT – 2l = 0, where AT is the transpose of A and I is the identity matrix. The value of
and A + AT – 2l = 0, where AT is the transpose of A and I is the identity matrix. The value of 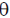 (in degrees) is ............................
(in degrees) is ............................
Ans. (90)
Sol. 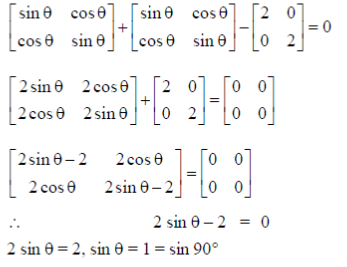
44. Truth table of a logic gate is given below
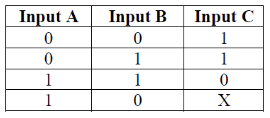
The value of X in the above table is .........................
Ans. (1)
Sol. In digital electronics, a NAND gate (NOT–AND) is a logic gate which produces an output which is false only if all its inputs are true, its output is complement to that of an AND gate. A low (0) output results only if all the inputs to the gate are HIGH (1), if any input is Low (0), a HIGH (1) output results.
Therefore, the value of X in the above table is 1.
45. Consider two particles, each of mass 20 g; the first particle is moving with a speed of 10 m/s along a one-deimensional track in the positive x-direction and collides with the second particle at rest. Assuming that the collision is elastic, the speed (in m/s) of th first particle after the collision is .......................
Ans. (0)
Sol. Vf1 = 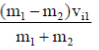
Vf1 = velocity of first particle after collison
m1 = mass of first particle
m2 = mass of second particle
vi1 = velocity of first particle before collison
Vf1 = 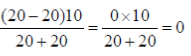
46. In the following reaction, the values of △H and △S at temperature 25°C are –13.7 kcal/mole and –16.0 cal/K.mole, respectively.

The value of 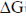 (in kcal/mole) of the reaction, rounded off to two decimal places, is ......................
(in kcal/mole) of the reaction, rounded off to two decimal places, is ......................
Ans. (–8.90)
Sol.
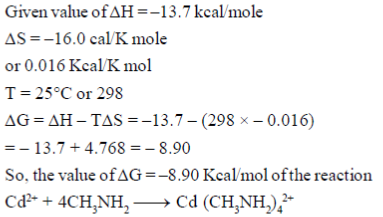
47. The volume (in mL) required to prepare 350 mL of 1X buffer solution from a fifty times (50X) concentration buffer stock solution is ........................
Ans. (7)
Sol. C1 × V1=C2 × V2
where,C1=Initial concentration
V1=Initial volume
C2=Final concentration
V2=Final volume
After putting the value
(50x) × V1=(1x) × 350 mL
V1=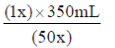
V1=7 mL
48. A compound microscope has its objective with linear magnification of 10. In order to achieve a final magnification of 100, the angular magnification of the eyepiece should be ............................
Ans. (10)
Sol. In order to ascertain the total magnification when viewing an image with a compound light microscope, take the power of the objective lens which is at 4x, 10 or 40x and multiply it by the power of eyepiece which is 10x.
Therefore, let angular magnification = x
Final magnification=100
Objective lens=10
10 × x=100
x=100/10
x=10
49. The decimal reduction time (DRT or D value) of a bacterial culture is one minute. If a suspension of the bacterial culture contains an initial population of 106 cells, then the time (in minutes) required to reduce the number of bacteria to 10 by heat treatment is ............................
Ans. (5)
Sol. t = D × (log N0 – log Nf)
Where,
D = D-value (min) at specified conditions
No = bioburden of the chosen bacterium at a given time
Nf = surviving population after an exposure time
So, replacing the values,
t=D × (log 106 – log 10)
t=1 (log 106 – log 10)
=6 log 10 – log 10
t=6 – 1 (log 10 – 1)
t=5 min
50. The median of Y in the following data is .............................

Ans. (15)
Sol. The ascending order of Y series is
10, 12, 14, 16, 20, 22
Here, n = 6 (which is even)
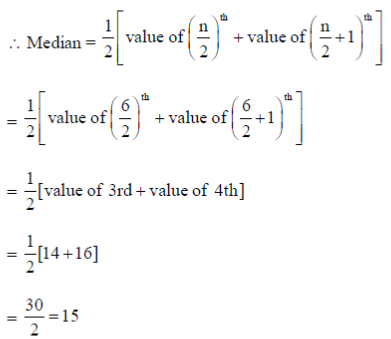
51. A Variable number of Tandem Repeats (VNTRs) locus has 15 different alleles. The number of genotypes possible in a populatioin for this VNTR is .......................
Ans. (120)
Sol. The number of genotype can be calculated by using the equation
Number of genotype = 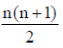
Where n is equal to the number of possible alleles.
Using the preceding equation, we have
Number of genotype = 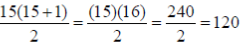
Therefore, for a locus having 15 different alleles, 120 different genotypes are possible.
52. The vibrational frequency (expressed in wavenumber) of 1H35 Cl is 2990.6 cm–1. Assuming that the force constant is same in both the cases, vibrational frequency (in cm–1) of 2D35Cl is ....................
Ans. (2143.87)
Sol.

53. The length of transverse and conjugate axis in a hyperbola are 6 and 8, respectively. The eccentricity of the hyperbola, rounded off to two decimal places is .........................
Ans. (1.66)
Sol. Length of transverse = 2b = 6
Length of conjugate axis = 2a = 8
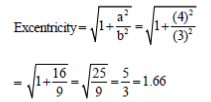
54. The solution to the limit 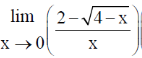 is ....................
is ....................
Ans. (0.25)
Sol.
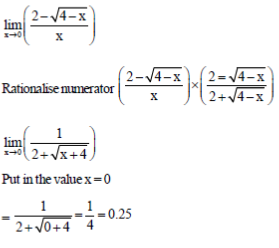
55. The average value of function f(x) - 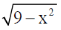 on [–3, 3], rounded off two two decimal places, is .......................
on [–3, 3], rounded off two two decimal places, is .......................
Ans. (2.35)
Sol.
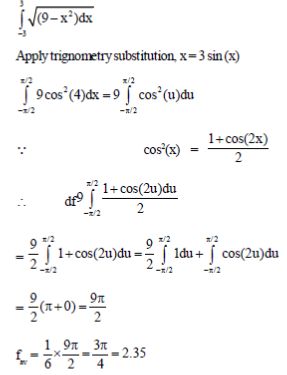
56. A bouncing ball is dropped from an initial height of meters above a flat surface. Each time the ball hits the surface, it rebounds a distance r × h meters and it bounces indefinitely.
Consider the value of h = 5 meters and r = 1/3. The total vertical distance (up and down) travelled (in meters) by the ball is ..........................
Ans. (10)
Sol. Height = h = 5m
after every bounce it goes to height = r × h
57. One point charge (q) each, is placed along a line at 3 different points x = 0, x = 2 nm and x = 6 nm. The force between two charges separated by 2 nm is piconewton (pN). The magnitude of force (in pN) on the charge in the middle due to the other two charges is ............................
Ans. (1.5)
Sol. f = 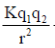
wheref=Force applied, K = Boltz constant
q1=Mass of first charge
q2=Mass of second charge
r=Distance between two charges
q1=q2 = q3 = 1
f1=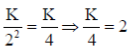
K=8
f2=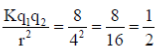
Final force=f1 – f2
=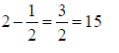
58. Energy of the electron in hydrogen atom in its ground state is 13.6 eV. The energy required (in eV) to move the electron from its ground state to the first excited state, rounded off to two decimal places, is ............................
Ans. (10.2)
Sol. En =  Ev [According to Bohr's model of H-atom]
Ev [According to Bohr's model of H-atom]
Energy required to excite from initial state to final state
E = Ef – Ei
Ef = Final energy state
Ei = Initial energy state
First excited state in n = 2
Ground state n = 1
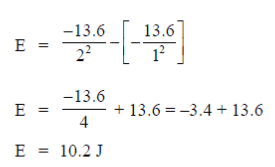
59. At a given time t, velocity (v) and acceleration (a) of a particle undergoing simple harmonic motion are given by

Assuming all quantities are in SI units, the amplitude of the oscillation is ......................
Ans. (5)
Sol. At a given time t,
Velocity is given by
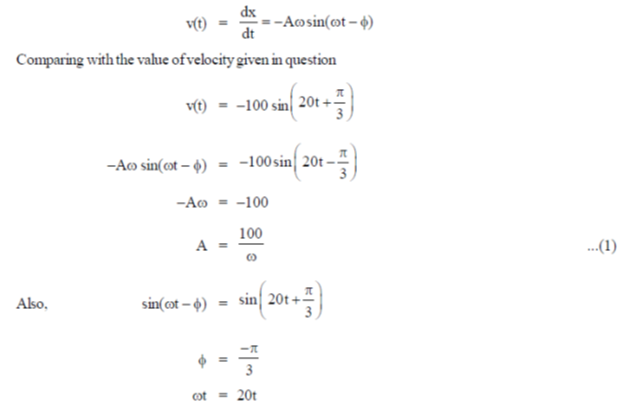
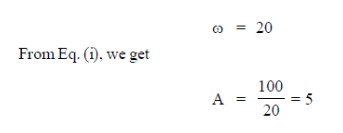
60. In an enzyme catalyzed first-order reaction, the substrate conversion follows an exponential pattern such that 80% of the substance is converted in 10 minutes. The first-order rate constant (in min–1) of the reaction, rounded off to three decimal places, is ...................
Ans. (0.167)
Sol.