IIT JAM Biology 2014
Previous Year Question Paper with Solution.
1. Match the proteins listed in Column in I with their major cellular functions in Column II.

(a) A-1, B-3, C-4, D-2
(b) A-2, B-3, C-1, D-4
(c) A-3, B-1, C-4, D-2
(d) A-2, B-1, C-4, D-3
Ans. (c)
Sol. Different proteins and their roles in molecular biology are :
(a) Tata binding protein-Transcription (general transcriptino factor that binds specifically to DNA sequence called TATA box.)
(b) DNA primase-Replication (type of RNA pol. which creates RNA primer)
(c) Aminoacyl RNA synyhetase-Translation (enzyme which catalyses the esterification of a specific cognate amino acid)
(d) RecA-Recombination (enzyme essential for the repair and maintenance of DNA
2. Amongst the following, the enongated fibrous protein is
(a) myoglobin
(b) keratin
(c) albumin
(d) calmodulin
Ans. (b)
Sol. A fibrous protein is the one being elongated in shape. It is meant to provide structural support for cells and tissues. These are also known as sclero proteins. They are generally water soluble. Alpha keratin and collagen have the sperical types of helices being found in them. They are also meant to serve a structural role in the human body. Among the options given, keratin is a family of fibrous proteins. It acts as a key structural material making up the outer layer of human skin, hair and nails. Hence, eventually meat to provide the necessary strength and toughness.
3. The mutation likely to cause the least perturbation in the tertiary structure of a protein is
(a) lysine is aspartate
(b) lysine to valine
(c) asparatate to glutamate
(d) aspartate is isoleucine
Ans. (c)
Sol. The correct match are as follows :
(a) Circular dichroism is an excellent tool for rapid determination of the secondary are folding propertyi3es of proteins that have been obtained using recombinant techniques or purified from tissues.
(b) lon exchange chromatography is a process that allows the separation of ions and polar molecules based on their affinity ot the ion exchange. It is often used in protein purification, water analysis and quality control.
(c) Immunoprecipitation, water a popular technique to identify physiologically relevant protein interactions by using target protein-specific antibodies to indirectly capture proteins that are bound to a specific target protein.
(d) X-ray crystallography is a form of very high resolution microscopy. It enables us to visulaise protein structure at the atomic level and enhances out understanding of protein function.
4. Match the techniques in Column I with their primary applications in Column II.
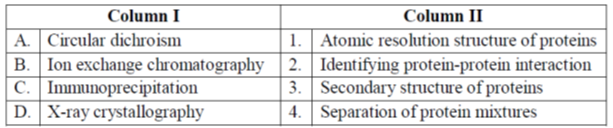
(a) A-2, B-4, C-1, D-3
(b) A-2, B-4, C-3, D-1
(c) A-3, B-1, C-4, D-2
(d) A-3, B-4, C-2, D-1
Ans. (d)
Sol. Correct answer is (d)
5. Amongst the following statements about biological membranes, the incorrect one is that they
(a) are covalent assemblies of lipids and proteins
(b) form selectively permeable barries
(c) may have channels and pumps
(d) show fluid-like behaviour
Ans. (a)
Sol. Biological membrane are dynamic structures being composed of a set of phospholipid molecules and proteins. They form a selectively permeable barrier among cells, i.e., permits only the selective molecules and ions to pass through them on the basis of the size, charge and other chemical properties. They may also have channels and pumps. The interactions of lipids, especially the hydrophobic cells, determines the lipid bilayer physical properties such as fluidity. These are used in the communication and transportation of chemicals and ions.
6. The introduction of a new fish species into a lake resulting in the extinction of several native fish species from the lake, is an example of
(a) co-extinction
(b) alien species invasion
(c) over-exploitation
(d) habitat loss
Ans. (b)
Sol. The phenomenon of introduction of alien or non-native (exotic) species results in the extinction of native species from the particular ecosystem. This is turn results in the loss of biodiversity. Invasive alien species occurs in all taxonomic groups including animals, plants, fungi and other microorganisms. Ecosystems been invaded by alien species may not have the natural predators and competitors present in its native environment that would normally control their operations. Islands are especially vulnerable to invasive alien species because of their natural isolation from strong competitors and predators e.g. Nile perch, Carrot grass, African cat fish, etc.
7. The taxonomic hierarchy in descending order of size is
(a) family, class, phylum, order
(b) phylum, class, order, family
(c) class, phylum, family, order
(d) order, family, class, phylum
Ans. (b)
Sol. In biological science, a taxon (pl. taxa) is known to be the group of one or more populations of an organisms that have been seen by taxonomists to form a unit. The term 'taxon' was first introduced by ICBN during 1956. In taxon individuals of one group have certain distinct characters from those of other groups. In taxonomic category, the complete arrangement is done from kingdom to species differently in both plants and animals. The decreasing order of taxonomic hierachy is given below :
Kingdom 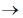 phylum
phylum 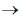 class
class 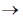 order
order  family
family  genus
genus  species
species
8. If the recessive disease phenylketonuria (PKU) occurs in a genetically constant population with a frequency of 1 in 10000, the frequency of the carrier genotype is
(a) 0.99%
(b) 19.9%
(c) 1.99%
(d) 9.9%
Ans. (c)
Sol. Phenyl ketonuria (PKU) is an autosomal recessive trait found rarely in individuals who lacks an enzyme phenylalanine hydorxylase, needed to breakdown an essential amino acid phenylalanine to tyrosine in the liver. According to the question if PKU as a disease has the low frequency of occurrence i.e. 1 in every 10,000 individual. Then the frequency of carrier genotype will be calculated as :
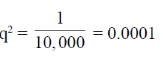
The square foot is 0.01 (equals to the frequency of PKU allele)
Frequency of normal allele will be = 1 – 0.01 = 0.09
Hence, the frequency of heterozygous genotype will be 2 × 0.09 × 0.01 = 0.198
This proves that about 2% of the population is a carrier of PKU allele. Hence, the requried percent is 1.99%
9. Amplification of a DNA fragment by PCR yields only one faint band of the expected size on an agarose gel. For such a sample, the best way to increase the yield of the PCR product is to
(a) decrease magnesium ion concentration
(b) decrease annealing temperature
(c) use shorter primers
(d) increase extension time
Ans. (b)
Sol. By decreasing annealing temperature, we can increase the yield of the PCR product. In a PCR reaction, each primer is a population of molecules and each strand of the template DNA is a population of molecules. Any one molecule of primer may stick anywhere on a molecule of template DNA whenever hydrogen bonds can form. The duration of annealing will depend on the temperature and on the number of hydrogen bonds that form between the primer and template molecules. A lower annealing temperature may increase the number and duration of mismatched associations, thereby increasing the probability that mismatched primers will get extended. At the end of the PCR programme, a PCR tube will contain the desired product but also all kinds of crap.
10. The cellular organelle which function(s) as a store for Ca2+ ions is
(a) endoplasmic reticulum
(b) Golgi bodies
(c) endosomes
(d) nucleus
Ans. (a)
Sol. The endoplasmic reticulum (ER) is a complicated system of membranous channels and flattened vesicles. It tends to be physically continuous with the outer membrane of the nuclear envelope. It occurs in most types of eukaryotic cells but are found to be absent in sperm cells and erythrocytes of human blood. They are of two main types, i.e., Rough ER (bearing ribosomes on their surface) and smooth ER (not bearing ribosomes on their surface). Our skeletal muscle is made up of number of muscle fibres bounded with sarcolemma (plasma membrane) and a well developed SR (i.e. sarcoplasmic reticulum), specialised for calcium storage in its sarcoplasm (cytoplasm).
11. If the N-terminal 21 amino acids were missing from a mitochondrial protein, its cellular location after synthesis would be
(a) mitochondria
(b) cytosol
(c) nucleus
(d) plasma membrane
Ans. (b)
Sol. Most mitochondiral proteins are encoded in the nucleus. They are synthesised as precursor forms in the cytosol and must be imported into mitochondria with the help of different protein translocases. Indeed, about 10% of the proteins in eukaryotic cell are imported into mitochondria. The problem becomes more complex as mitochondrial proteins reside in four locations, i.e., membrane, inner membrane, intermembrane space and the matrix.
It was discovered that the import of protein into the mitochondria requires the presence of a particular sequence as its amino or N-terminal end called presequence on matrix targetting sequence, which is being recognised by receptors situated on the external face of the outer membrane of mitochondrion and leads of the import of protein bearing it into the matrix. Thus, if any of the N-terminal amino acid will be missing from a mitochondrial protein, the protein will remain in the cytosol after being synthesised.
12. Packaged biomaterials are dispatched to intracellular and extracellular locations from the
(a) 1, 2 and 2
(b) 2, 2 and 1
(c) 2, 1 and 2
(d) 1, 2 and 1
Ans. (c)
Sol. During the process of glycosylation of proteins at the level of ER, proteins are transported from the ER to the Golgi complex in transport vesicles. Golgi complex is an asymmetric stack of flattened membranous sacs called cisternae. The golgi complex is differentiated into a cis-compartment which is known as the receiving compartment, a medial compartment and trans-compartment from which proteins and other packaged biomaterials are dispatched to the intracellular ad extracellular locations or to the various destinations.
13. The prefered ligand for SH2 domains is
(a) serine-phosphorylated peptide
(b) tyrosine-phosphorylated peptide
(c) glucose-6-phosphate
(d) cyclic AMP
Ans. (b)
Sol. One of the most prominent domain the protein-protein interactions which plays a major role in cellular growth and development is SH2 domain. It plays a vital role in the cellular communication. The length of SH2 domain is approximately 100 a.a long. Research has shown that SH2 domain has a high affinity to phosphorylated tyrosine residues and it is known to identity a sequence of 3-6 amino acids within a peptide motif.
14. The binding of a hormone to its receptor activates adenylyl cyclase through a stimulatory G-protein. if, due to a mutation, the G-protein binds but does not hydrolyse GTP, the consequence will be
(a) adenylyl cyclase will be continuously activated
(b) adenylyl cyclase will never to activated
(c) adenylyl cyclase will be occasionally activated
(d) adenylyl cyclase will be degraded
Ans. (a)
Sol. During a mechanism of hormone action of a peptide hormone, which interacts with the membrane bound receptors does not enters their target cell in normal condition, they instead generates their secondary messangers to regulate the metabolism of the body. During the action, a peptide hormone being water soluble in nature binds to the extrinsic receptors (on the cell causing) the release of an enzyme called adenylate cyclase, which leads to the formation of cAMP from ATP in the cell from the receptor site. If due to any mutation, the G-protein gets bind but fails to hydrolyse GTP, the enzyme adenyl cyclase will remain activated for continuous.
15. A toxin which causes accumulation of twice the normal amount of DNA in a dividing mammalian cell, most likely blocks the cell cycle
(a) during G0-phase
(b) after G1-phase
(c) after M-phase
(d) during G2-phase
Ans. (d)
Sol. Cell cycle is an orderly sequence of events by which a cell duplicates its genome, synthesises the components important for the cell eventually dividing into two daughter cells. For this, each and every cell follows a set of stages such as interphase (i.e. G1, S and G2 phase) and M-phase (mitotic phase). For the occurrence of protper and controlled division of the cell, all phases are have cell cycle check points to control mechanisms in eukaryotic cells. Each checkpoint acts as a potential halting point along the cell cycle.
The different checkpoints are G1 (start) checkpoint, G2/M check point, Metaphase check point. If by mistake in a typical dividing mammalian cell, the normal amount of DNA become twice due to an accumulation of some toxin, the cycle will halt during G2 phase, by the G2/M checkpoint also known as DNA damage checkpoint which ensures that the cell underwent all of the necessary changes during S and G2 phases and is ready to divide.
16. Bt toxin produced by Bacillus thuringinesis, does not kill the bacteria itself bacause the toxin is
(a) isolated in a special intracellular sac
(b) in an inactive form inside the bacterial cell
(c) active only against eukaryotic ribosomes
(d) produced in very small quantities
Ans. (b)
Sol. Bt toxin is produced by a bacterium called Bacillus thuringinesis, which produces a protein crystals (Cry) during a particular phase of their growth. These crystals contain a toxic insecticidal protein. Bt toxin protein exists as an inactive protoxin, but once an insects ingest it, its inactive from oncerts into an active form due to the alkaline pH of the gut that solubilises the crystals.
17. The inactivation of an mRNA due to its binding to a complementary RNA molecule is called
(a) RNA interference
(b) RNA splicing
(c) RNA translation
(d) RNA looping
Ans. (a)
Sol. RNA interference is a biological process in which the RNA molcules inhibits the gene expression. This method typically involves the silencing of a specific mRNA due to a ciomplementary dsRNA moelcule which binds and prevents the ranslation of mRNA.
18. Given are the sequences of one strand of double-stranded DNA. The one with the highest melting point (Tm) is
(a) GAGATCTCGAGATCTC
(b) GAGATCTTGATATCTC
(c) GAGATATCGATATCTC
(d) GAGATATCTATATCTC
Ans. (a)
Sol. Melting point of DNa is related to the GC content of it. Since, GC forms hree hydrogen bonds while AT forms two hydrogen bonds. Thus, the more the GC content in the sequence of DNA, the higher will be the melting point. Thus GAGATCTCGAGATCTC has the highest melting point.
19. The standard pregnancy kit, used to detect human Chorionic Gonadotrophin (hCG) in urine, is based on
(a) gene amplification through PCR
(b) antigen-antibody interaction
(c) biotin-streptavidin interaction
(d) nickel affinity chromatography
Ans. (b)
Sol. Modern urine pregnancy testing kits are based on the detection of human chorionic Gonadotrophin (hCG) by immunological methods. hCG is a hormone produced by placenta and is found in the serum and urine of the pregnant women shortly after fertilisation. During the examination, the hCG in the specimen (the antigen) interact with the anti hCG, a commercially prepared antibody specific for the hormone.
20. The preferred system for large-scale production of influenza virus for vaccination is
(a) genetically modified bacteria
(b) transgenic plant
(c) chick embryo
(d) yeast culture
Ans. (c)
Sol. Before the development of cell culture, many viruses were propagated in the embryonated chicken eggs. Today this method is most commonly used for growth of influenza virus. The excellent yield of virus from chicken eggs has led to their widespred use in research laboratries and for vaccine production. For propagation of influenza virus, pathogen-free eggs are used 11-12 days after fertilisation. After the complete process of propagation, during incubation period, the virus replicates in the cells that makes up the chorioallantonic membrane.
21. A monoclonal antibody produced against a small peptide derived from protein X, is unable to bind X in an ELISA. This is because
(a) peptide antibodies do not bind to immobilised proteins
(b) the peptide epitope is exposed in X
(c) monocolonal antibodies cannot be used in ELSIA
(d) the peptide epitope is buried in the interior of X
Ans. (d)
Sol. Antibodies are proteins produced by our immune system in response to foregin proteins i.e., antigen.
Antibodies functions as markers, binding to the antigen so that the antigen molecule can be recoginsed and destroyed by phagocytes. The part of the antigen that the antibody binds in called the epitope. The two features i.e. specificity (the antibody binds only to its particular epitope) and sufficiency (the epitope can bind to the antibody on its own) are key to the use of monoclonal antibodies as a molecular tool.
22. The lac repressor is produced from a stretch of DNA called the
(a) regulator
(b) operator
(c) promoter
(d) inducer
Ans. (a)
Sol. Lac-repressor is a DNA-binding protein which is meant to inhibit the expression of lactose in bacteria. It is synthesised from a stretch of DNA called regulator. The repressor from a stretch of DNA called regulator. The repressor protein binds to the operator region to keep RNA polymerase from transcribing lac genes.
23. The repeating units in chitin are
(a) 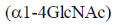
(b) 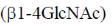
(c) 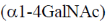
(d) 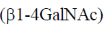
Ans. (b)
Sol. Chitin is a long complex polysaccharide made up of repeating units of disaccharide acetyglucosamine by the 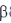 -1, 4 linkage (
-1, 4 linkage (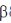 -1-4 GIC NAc). It is similar to cellulose (made up of glucose). But chitin has different chemical groups coming off its own unique function. Functionally, chitin is one of the major components of the cell wall of fungi and exoskeleton of insects and arthopods.
-1-4 GIC NAc). It is similar to cellulose (made up of glucose). But chitin has different chemical groups coming off its own unique function. Functionally, chitin is one of the major components of the cell wall of fungi and exoskeleton of insects and arthopods.
24. The correct ascending order of melting points of oleic acid (O), linoleic acid (L), palmitic acid (P) and stearic acid (S) is
(a) L, O, P, S
(b) O, L, P, S
(c) L, O, S, P
(d) O, L, S, P
Ans. (a)
Sol. The lipids are large and diverse group of naturally occuring organic compounds that are related by their solubility is non-polar solvents. Natural fatty acid may be either saturated and unsatruated. The saturated fatty acids have higher melting points than unsaturated acids of higher melting points than unsaturated acids of corresponding size. Thus, the sequence of ascending order of melting points is L, O, P, S (i.e. 5°C, 13°C, 63°C, 69°C).
25. A peptide Glu-His-Trp-Ser-Gly-Leu-Arg-Pro-Gly, having an isoelectric point of 7.8, is placed in an electric field at pH 3.0. It will migrate towards
(a) anode
(b) cathode
(c) Both (a) and (b)
(d) Neither anode nor cathode
Ans. (b)
Sol. Isoelectric focusing is an electrophoretic method in which proteins are separated on the basis of their PIs. It actually makes use of the property of proteins that their net charges are determined by the pH of their local environments. Proteins inturn carries both positive, negative or even zero net electrical charge depending on the pH of their surroundings.
Proteins are positively charged in solutions at pH values below their pI and negatively charge at pH values above their PI. Thus, the amino acids present in the peptide given in the question, i.e. Glu-His-Trp-Ser-Gly-Leu-Arg-Pro-Gly have their PI values more than the pH(3) of the solution (i.e. pH value is below PI values). Hence a particular peptide or protein will move towards cathode.
26. X-ray diffraction of wool shows repeated structural units spaced at 5.2Å which is changed to 73.0 Å on steaming. This is due to the conversion of secondary structure from
(a) 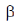 -sheet to random coil
-sheet to random coil
(b) 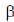 -helix to random coil
-helix to random coil
(c) 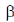 -sheet to
-sheet to 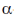 -helix
-helix
(d) 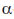 -helix to
-helix to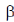 -sheet
-sheet
Ans. (d)
Sol. Early X-ray diffraction studies of fibrous proteins such as hair and wool, showed a major periodicity or repeat unit of 5.0-5.5 Å indicating some regularity in the structure of these proteins. The 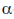 -helix is a rod-like structure which is stablised by hydrogen bonds between the NH and CO groups of the main chain.
-helix is a rod-like structure which is stablised by hydrogen bonds between the NH and CO groups of the main chain.
Thus, the 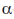 -helical structure depends on the intramolecular hydrogen bonding. It has a pitch of 5.4 Å containing 3.6 amino acids per turn of the helix. The
-helical structure depends on the intramolecular hydrogen bonding. It has a pitch of 5.4 Å containing 3.6 amino acids per turn of the helix. The 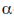 -helix of hair or wool shows an interesting phenomenon when they are tredated with moist heat (steaming) and stretched by converting into
-helix of hair or wool shows an interesting phenomenon when they are tredated with moist heat (steaming) and stretched by converting into 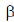 -sheets because α-keratins gets converted into
-sheets because α-keratins gets converted into 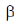 -keratin (7Å diameter)
-keratin (7Å diameter)

27. At Et = 20 nm and substrate concentration = 40 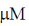 , the reaction velocity V0 of an enzyme is 9.6
, the reaction velocity V0 of an enzyme is 9.6 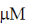 s–1. Assuming kcat to be 600 s–1, the KM will be
s–1. Assuming kcat to be 600 s–1, the KM will be
(a) 0.1 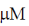
(b) 1 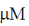
(c) 10 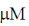
(d) 100 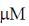
Ans. (c)
Sol. According to Michaelis-Menten equation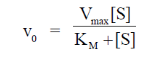
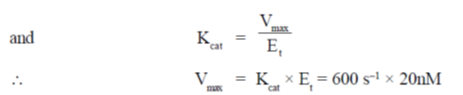

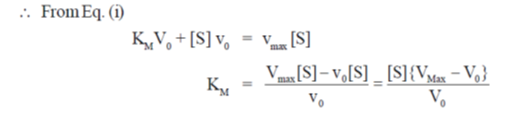
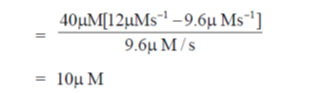
28. Which of the following statements is NOT true for an enzyme catalyzed reaction?
(a) Reaction rate and equilibrium both are altered
(b) Activation energy is decreased
(c) Enzyme-substrate complex is formed
(d) Enzymes undergo
Ans. (a)
Sol. Enzymes are efficient catalysts for biochemical reactions. They speed up reactions by providing an alternative reaction pathway of lower activation energy. They can only alter rate of reaction, not the position of the equilibrium.
The substrate simply fits into the active site of enzyme to a reaction intermediate i.e., enzyme-substrate complex.
Enzyme + Substrate  Enzyme-substrate complex
Enzyme-substrate complex
29. Which of the following is NOT an allosteric regulatory enzyme in glycolysis?
(a) Hexokinase
(b) Phospho-fructokinase I
(c) Phosphoglycerate kinase
(d) Pyruvate kinase
Ans. (c)
Sol. Phosphoglycerate kinase (PGK) is an enzyme responsible for catalysing the reversible transfer of high energy phosphate group in glycolysis from 1, 3 BPG to ADP by producing 3-PG and ATP. This is the first ATP generating step in glycolysis.
It is the only enzyme of glycolysis which is not an allosteric regulated enzymes because it does not requires adivalent metal cofactor. Rest all three are the enzymes for allosteric regulation because they requires cofactors such as Mg2+, etc. The concentration of these three enzymes in the cell is been regulated by the hormones that affects the rate of transcription.
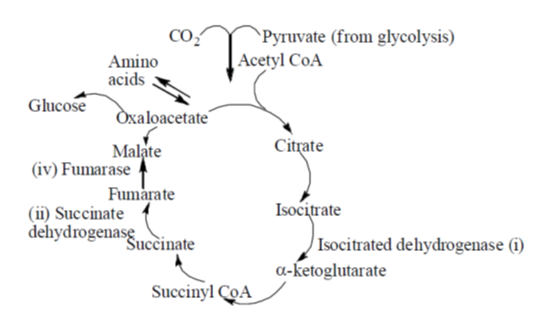
30. Match the enzymes of TCA cycle in Group I with that of their products listed in Group II.

(a) P-1, Q-2, R-4, S-3
(b) P-1, Q-2, R-4, S-3
(c) P-2, Q-4, R-3, S-1
(d) P-1, Q-3, R-4, S-2
Ans. (d)
Sol. TCA or Tricarboxylic acid cycle. citric acid cycle (Krebs' cycle) is the most nearly univesal pathway for aerobic metabolism. It is a series of reactions occuring in the mitochondria of the cell that brings the complete oxidaton of acetyl Co-A to CO2 liberating hydorgen equivalents that ultimately forms water. Different enzymes involved in the cycle are given below.
31. Addition of the uncoupler 2, 4-Dinitrophenol to activity respiring mitochondria causes
(a) decrease in ATP production and increased rate of O2 consumption
(b) decrease in ATP production and decreased rate of O2 consumption
(c) increase in ATP production and increased rate of O2 consumption
(d) increase in ATP production and decreased rate of O2 consumption
Ans. (a)
Sol. 2, 4 Dinitrophenol (DNP), introduced by loomis and lipmann is one of the most effective agents for uncoupling respiratory chain phosphorylation. It does not have any effect on substrate level phosphorylation taking place in glycolysis. It's uncoupling action is due to te fact that both phenol and corresponding phenolate ion are significantly soluble in the lipid core of the inner mitochondrial membrane. DNP also stimulates the activity of an enzyme ATPase (normally inactive as a hydrolytic enzyme in mitochondria), Actually ATP is nevr formed in the presence of DNP, since the high energy intermediate is attacked, i.e., acts prior to the step ATP synthesis. Thus, production i.e., acts prior to the step of ATP synthesis. Thus, production of ATP decreases and the rate of O2 consumption gets increased.
32. C4 plants overcome photorespiration activity of Rubisco by fixing CO2, firstly as
(a) oxaloacetate
(b) 3-phosphoglycerate
(c) 2-phosphoglycerate
(d) ribulose 1, 5- bisphophate
Ans. (a)
Sol. Photorespiration is a light dependent cyclic process of oxygenation of RuBP and release of CO2 by the phottosynthetic organs of a plant. It takes place in the chloroplast of the plant cells.
33. Which of the following is a non-symbiotic nitrogen fixing bacteria?
(a) Rhizobium leguminosarum
(b) Nitrosomonas nitrosus
(c) Azotobacter chrococcum
(d) Alcaligenes faecalis
Ans. (c)
Sol. Non-symbiotic or free-living nitrogen fixing bacterias are those bacteria that are not present in close relationship with another bacteria of other organisms in order to convert nitrogen into ammonia Azotobactor in order to convert nitrogen into ammonia. Azotobactor chrococcus is one of the non-symbiotic aerobic bacteria that have the highest known rate of respiratory metabolism of any organism, so they might protect the enzyme by maintaining a very low level of O2 in their cells. They are also known to have copious amounts of extracellular polysaccharide.
34. Vasopressin, an antidiuretic hormone, responsible for increased absorption of water by the kidney, is secreted from
(a) adrenal gland
(b) thyroid gland
(c) pituitary gland
(d) parathyroid gland
Ans. (c)
Sol. Vasopressin or Anti-Diurectic Hormone (ADH) is a nine amino acid peptide being secreted by the posterior lobe of the pituitary gland. The single most important effect of ADH is to conserve water by reducing the loss of water in urine. Antidiuretic hormone binds to the receptors on cells in the collecting ducts of kidney promoting reabsorption of water back into the circulation. In the absence of ADH, the collecting ducts are virtually impermeable to water and it flows out as urine.
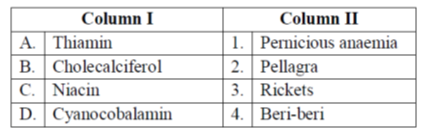
35. Match the vitamins in Group I with their deficiency disorders listed in Group II.
(a) P-1, Q-2, R-3, S-4
(b) P-4, Q-3, R-2, S-1
(c) P-2, Q-4, R-1, S-3
(d) P-3, Q-1, R-4, S-2
Ans. (b)
Sol. A vitamin is an organic compound and a vitual nutrient that an organism requires in limited amounts. They are not synthesised. Deficiency of vitamins in body may result in one or other type of deficiency in the body Different vitamins and its deficiency disorder is
(a) Thiamin (Vit-B1) – Beri-beri
(b) Cholecalciferol (Vit-D) – Rickets
(c) niacin (Vit-B3) – Pellagra
(d) Cyanocobalamin (Vi-B12) – Pernicious anaemia
36. Which of the following enzymes are secreted by pancreas?
P. Pepsin
Q. Aminopeptidase
R. Trypsin
S. Carboxypeptidase
T. Chymotrypsin
(a) P, Q, R
(b) Q, R, T
(c) R, S, T
(d) P, R, T
Ans. (c)
Sol. Pancreas is an elongated organ situated partly behind the stomach between the limbs of duodenum. An alkaline pancreatic juice containing enzyme is secreted by the exocrien portion of pancreas. It contains various inactive enzymes such as chymotrypsin, procarboxypeptidases and trypsinogen which are activated chymotrypsin by different enzymes and are converted into carboxypeptidase and trypsin respectively.
37. Which part of the human brain controls body temperature?
(a) Cerebrum
(b) Medulla
(c) Cerebellum
(d) Hypothalamus
Ans. (d)
Sol. The hypothalamus is the portion of the brain that contains a number of small nuclei with a variety of functions. It is located bwlow the thalamus. It is responsible for certain metabolic processes and other activites of autonomic nervous system. It helps to control body temperature, hunger, thirst, fatigue, sleep and cardiac rhythms.
38. The action potential for initiating and maintaining the rhythmic contraction of heart is generated by
(a) sino-atrial node
(b) atrio-ventricular node
(c) bundle of His
(d) atrio-ventricular bundle
Ans. (a)
Sol. The SA node or sinus node is the heart's natural pacemaker. It consists of cells that are situated in the upper part of the wall of the right atrium. It is responsible for the initiation of the heart beat. It spontaneously generates an electrical impulse which after conducting throughout the heart, causes the heart to contract.
39. The antigen binding sites in immunoglobulin IgG are present at
(a) variable region of heavy chains
(b) variable region of light chains
(c) constant region of heavy chains
(d) variable region of heavy and light chains
Ans. (d)
Sol. IgG is a type of antibody composed four peptide chains, i.e., two identical light and heavy chains. Each IgG has two antigen binding sites, i.e. at the variable regions of both heavy and light chains. It represents approximately 75% of serum antibodies. It is the most common type of antibody found in circulation of humans. The molecules of IgG are created and released by plasma B-cells.
40. Fertilization of human sperm and ovum takes place in the
(a) ovary
(b) uterine cavity
(c) fimbria-infundibulum
(d) isthamus-ampulla
Ans. (d)
Sol. Fertilisation is the moement when a sperm and egg joined together and the genes from the mother and father combine to form a new life. During a process, the motile sperms move through the cervix enters the uterus and reaches the junction of the isthmus and ampulla (ampullary isthmic junction) of the Fallopian tube where fertilisation takes place.
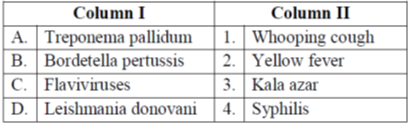
41. Match the pathogenic microorganisms in Group I with the diseases listed in Group II.
(a) P-1, Q-4, R-3, S-2
(b) P-4, Q-1, R-2, S-3
(c) P-4, Q-2, R-3, S-1
(d) P-1, Q-3, R-2, S-4
Ans. (b)
Sol. Symphilis is an infectious venereal disease caused by Treponema pallidum, whooping cough is a contagius bacterial disease caused by Bordetella pertusis chiefly affecting children being characterised by convulsive coughs followed by a whoop. Yellow fever is an acute viral disease transmitted by infected mosquitoes bite of Flavi viruses. Kala azar is a vector borne disease transmitted by the bite of sandfly genus leishmania donovani.
42. An example of a eukaryotic chemoorganotrioph microorganism lacking chlorophyll and having mycelial thallus is
(a) yeast
(b) bacteria
(c) fungi
(d) protozoa
Ans. (c)
Sol. Fungi are eukaryotic chemorgantrophic microorganisms synthesising the organic compounds they need for growth and energy from pre-existing organic sources in their environment using the energy from chemical reactions. They lack chlorophyll and have mycelial thallus.
43. A Bacillus sp. divides every 30 min. If a culture is inoculated with 1000 cells, how many cells will be generated after 3 hrs?
(a) 30,000
(b) 64,000
(c) 90,000
(d) 128,000
Ans. (b)
Sol. If a Bacillus sp. grows and divides in every 30 min.
Then, the number of generations after 3 hrs will be 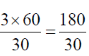 = 6 generations cycles
= 6 generations cycles
Now, in each 30 min, the number of cell will get doubled. If the number of cells inoculated in a culture are 1000
Then, the number of cells in 3 hrs
= 1000 × (2)6 = 64,000 cells
44. The selective media mannitol salt agar is used for the isolation of
(a) Lactobacillus
(b) Enterococcus
(c) Staphylococcus
(d) Salmonella
Ans. (c)
Sol. Mannitol Salt Agar (MSA) is commonly used both as a differential and a selective growth medium encouraging the growth of a group of certain bacteria while inhibiting the growth of others. It is mainly used for the isolation of Gram positive bacterium Staphylococcus as it contain high concentration of NaCl (i.e., 7.5%-10%) as this level is inhibitory to most other bacterias.
45. In liver cells, glucose is converted to glucose-6-P which can then be utilized towards glycolysis or glycogen synthesis. If KMGlycolysis and KMGlycogen correspond to the enzymes involved in the first steps of glycolysis and glycogen synthesis, the true statement amongst the following is
(a) Glycogen synthesis is favoured at high glucose concentrations if KMGlycolysis < KMGlycogen
(b) No glycogen is formed at high glucose concentrations if KMGlycolysis > KMGlycogen
(c) No glycolysis occurs at high glucose concentrations if KMGlycolysis < KMGlycogen
(d) Glycolysis is favoured at low glucose concentrations if KMGlycolysis > KMGlycogen
Ans. (d)
Sol. G6R is an important compound being at the junction of many metabolic pathways such as glycolysis, glycogenolysis, gluconegenesis and hexose monophosphate shunt. Gluconegenesis and glycolysis are coordinated so that within a cell one pathway is relatively inactive, while the other is highly active. Both are highly exergonic under celluar condition and so there is no thermodynamic barrier to such simultaneous activity. The rate of glycolysis is also determined by the concnetration of glucose and the rate of gluconeogenesis by the concentration of lactate and other precursors of glucose. Hence, glycolysis is nearly switched off and gluconegenesis is promoted.
46. An enzyme requries both asparatate (pKa of side chain = 4.5) and histidine (pKa of side chain = 6.5) residues in the catalytic site to be protonated for activity. The expected enzyme activity (in%) at a pH of 5.5 would be closest to
(a) 90
(b) 78
(c) 50
(d) 10
Ans. (d)
Sol. Given
pKa (Aspartate) = 4.5
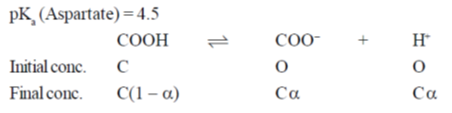
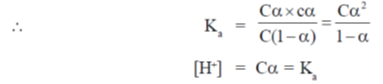
= Antilog (–pKa)
= Antilog (–4.5) = 3.16 × 10–5
pKa (Histidine) = 6.5
Similarly for histidine
[H+] = 3.16 × 10–7
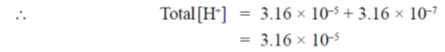
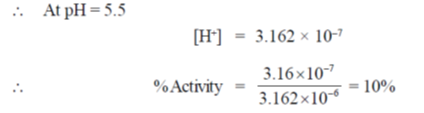
47. By weight, 95% ofan E. coli cell's components are water (~70%), protein (~15%), nucleic acids (DNA ~ 1% + RNA ~ 6%) and polysaccharides (~3%). Given that there is only one chromosome and about 3000 different proteins in an E.coli cell lysate, the number of different molecules of DNa and RAN is expected to be
(a) DNA = RNA = 3000
(b) DNA = RNA > 3000
(c) DNA = 1, RNA > 3000
(d) DNA > 3000, RNA = 1
Ans. (c)
Sol. In the given E.coli the number of DNA = 1 Because there is only one chromosome (strand of DNA that is encoded with genes) in the E.coli.
Also, proteins are formed after translation from mRNA. Therefore, for making 3000 proteins, many mRNA are required. In addition to that E.coli contains rRNA, tRNA snRNA etc. Thus, here number of RNA must be more than 3000.
48. Determine the correctness or otherwise of the following Assertion [a] and Reasion [r].
Assertion : The general trend across a period is an increase of the ionization energy
Reason : The potential energy of attraction between the electron and nucleus increases with the nuclear charge.
(a) Both [a] and [r] are true and [r] is the correct reason for [a]
(b) [a] is false but [r] is true
(c) Both [a] and [r] are true but [r] is NOT the correct reason for [a]
(d) Both [a] and [r] are false
Ans. (a)
Sol. As moving across the period in periodic table the size of atoms decreases due to increase in effective nuclear charge. It means that number of electrons and protons and increases so, attraction between nucleus and electrons also increases thus, ionisation energy increases i.e., more potential energy required to remove outermost electron.
49. The monomer which leads to a conducting polymer is
(a) but-2-yne
(b) E-but-2-ene
(c) Z-but-2-ene
(d) buta-1, 3-diene
Ans. (a)
Sol. Conductive polymers are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive polymers are generally not thermoplastics. They can offer high electrical conductivity but do not show similar, mechanical properties to other commercially available polymer.
But-2-yne is the monomer of conduction polymer.
50. The pH at the equivalence point when 50 mL of 0.1 M acetic acid is titrated against 0.1 M NaOH is closest to
(a) 6.0
(b) 7.0
(c) 8.0
(d) 9.0
Ans. (d)
Sol. CH3COOH + NaOH 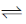 CH3COONa + H2O
CH3COONa + H2O
At equivalence point
Macid Vacid = Mbase Vbase
or 0.1 × 50 = 0.1 × Vbase
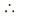 Vbase = 50 mL
Vbase = 50 mL
Since, Ka = 1.8 × 10–5 for acetic acid
Number of millimoles of acid and base at equivalence point
= 0.1 × 50 = 5 m mol
When, equimolar weak acid is titrated with strong base, then solution becomes slightly alkaline and pH is given as
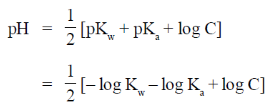
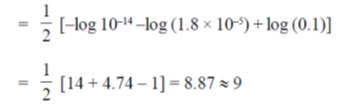
51. The mass (in g) of glycine, NH2CH2COOH, required to make 250 mL of 0.015 M solution is (Atomic weights in amu : H = 1, C = 12, N = 14, O = 6)
(a) 1.13
(b) 0.84
(c) 0.56
(d) 0.28
Ans. (d)
Sol. Molecular mass of glycine = 75g
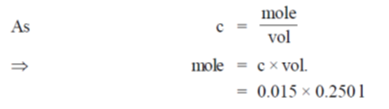

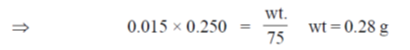
52. The arrangement of ligands in ascending order of the crystal field splitting is
(a) I– < H2O < OH– < CN–
(b) I– < H– < H2O < CN–
(c) H2O < OH– < CN– < I–
(d) H2O < CN– < I– < OH–
Ans. (b)
Sol. The ligands are classified as weak and strong ligands. The ligands which casue a small degree of splitting of d-orbitals are called weak ligands and the ligands whkch cause a large spittinh are called strong ligands. The common ligands have been arranged in order of there increasing crystal field splitting power as
I– < OH– < H2O < CN–
53. Determine the corrections or otherwise of the following Assertion [a] and the Reason [r]
Assertion : The boiling points of the group VIA (16) hydrides increase with size without exception
Reason : London dispersion forces increase with molecular weight
(a) Both [a] and [r] we true and [r] is the correct reason for [a]
(b) [a] is false but [r] is true
(c) Both [a] and [r] are true but [r] is NOT the correct reason for [a]
(d) Both [a] and [r] are false
Ans. (b)
Sol. The boiling point of group-16 hydride decrease with increasing size without exception
H2O > H2S > H2Se > H2Te
Because H2O form H-bonding with othe H2O molecules and formed associated form while other hydrides viz., H2S, H2Se and H2 Te exist in gas and has weak London disperison force which is weaker force than H-bonding force. So, the boiling point decrease.
54. Determine the correctness or otherwise of the following Assertion [a] and the Reason [r]
Assertion : Boiling points of aldehydes and ketones are higher than the boiling points of the corresponding eithers and lower than alcohols
Reason : The carbonyl group is polar but does not undergo intermolecular hydrogen bonding
(a) Both [a] and [r] are true and [r] is the correct reason for [a]
(b) [a] is false but [r] is true
(c) Both [a] and [r] are true but [r] is NOT the correct reason for [a]
(d) Both [a] and [r] are false
Ans. (a)
Sol. Boiling points of aldehydes and ketone are higher than the boiling points of the corresponding either and lower than alcohols because alcohols formed associated intermolecular H-bond and it is a covalent molecule but carboxyl group is polar it does not undergo intermolecular hydorgen bonding.
55. In the reaction sequence below, X, Y, Z, and Z, respectively are
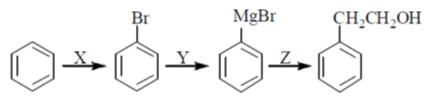
(a) Br2, FeBr3; MgCl2; CH2O, H+
(b) HBr; HgCl2; CH3CHO, H+
(c) Br2, hv; MgCl2; CH2O, H+
(d) Br2, FeBr3; Mg, THF;  ,H+
,H+
Ans. (d)
Sol. 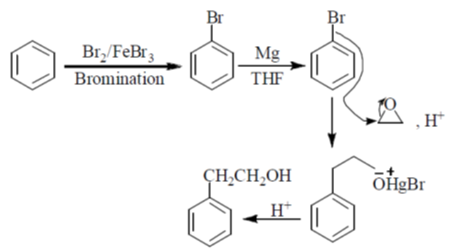
56. On completion of the reaction
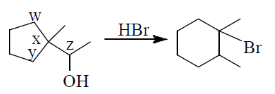
the Br atom is attached to carbon atom
(a) w
(b) x
(c) y
(d) z
Ans. (b)
Sol.
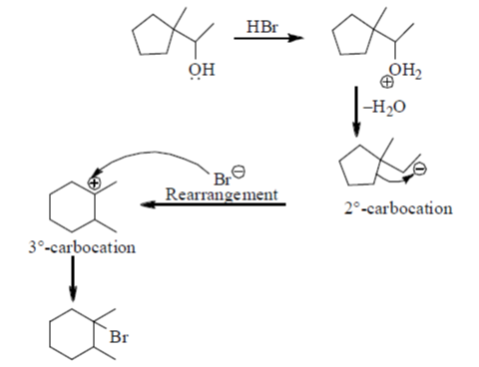
57. An aqueous is a mixture of a carboxylie and (pKa = 4.0) and an amine (pKa of protonated amine = 10.0). The separate the components, the solution at a pH of 2.0 is shaken with diethyl ether. On standing, the
(a) top water layer would contain the amine
(b) top ether layer would contain the amine
(c) top water layer would contain the acid
(d) top ether layer would contain the acid
Ans. (d)
Sol. In the process of solvent extraction, 2 solvents are taken (hydrophobic + hydrophilic) which are diethyl ether (low density, makes top layer) and solution of pH = 2 (high density of water, bottom layer). Hence, top later is of diethyl ether. On solvent extraction ,some of carboxylic acid and amine will be in ether, some of it will be in solution of pH = 2 :
In top layer of ether
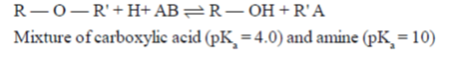
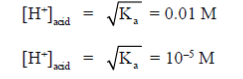
All the amine will react and excess of carboxylic acid will remain in diethyl ether.
58. The major product Z, obtained in the reaction is
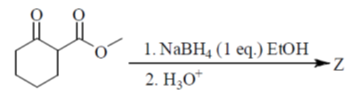
(a) 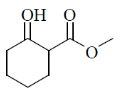
(b) 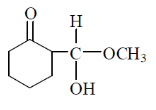
(c) 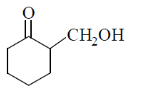
(d) 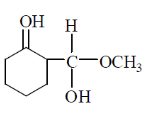
Ans. (a)
Sol.
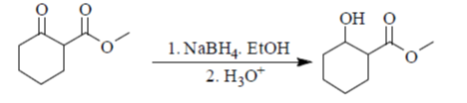
NaBH4 is a reducing agent for aldehyde and ketone. In this compound two groups are present ketonic and ester group. So, NaBH4 reduce only ketonic group to alcoholic group. It is a selective reducing agent.
59. The compound that shows a line in the 1HNMR spectrum, at the lowest  value is
value is
(a) CH2Cl2
(b) CHCl3
(c) CH3Cl
(d) CH3l
Ans. (d)
Sol. A proton is said to be deshielded if it is attached to an electronegative atom or group.
Greater the electronegativity of the atom, greater is the deshielding caused to the proton. If the deshielding is more for a proton, then its  -value will also be more.
-value will also be more.
F > Cl > Br > 1
Hence, CH3l shows a line in the 1H NMR spectrum at the lowest 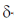 -value.
-value.
60. Water is injected into a balloon filled with ammonia gas. The ballon shrinks and it is hot to touch. According to the convention 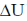 = q + w, for this process
= q + w, for this process
(a) q > 0, w > 0
(b) q > 0, w = 0
(c) q < 0, w > 0
(d) q < 0, w < 0
Ans. (c)
Sol. According to question, ballon shrinks which means V2 < V1
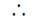 w = –p dV = (+) ve
w = –p dV = (+) ve
Also, heat is evolved from the ballon
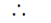 q = – ve
q = – ve
Thus, 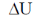 = –q + w
= –q + w
Only when, q < 0 and w > 0.
61. A process cannot occur spontaneously at constant T and P when
(a) 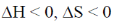
(b) 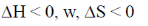
(c) 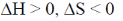
(d) 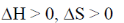
Ans. (c)
Sol. From Gibb's-Helmholtz equation, we have
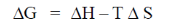
As process is non-spontaneous only when 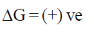
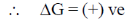
62. If an atomic orbital has 2 radial nodes and 1 angular node, it is a
(a) 2p orbital
(b) 3d orbital
(c) 3p orbital
(d) 4p orbital
Ans. (d)
Sol. Here, 2 radial node
1 angular node
Radial node s-orbital = n – 1
p-orbital = n – 2
d-orbital = n – 3
f-orbital = n – 4
Angular node 0 1 2 3
s p d f
If n = 4, then radial node
for p-orbital = n – 2 = 4 – 2 = 2 node
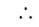 The atomic orbital is 4p-orbital.
The atomic orbital is 4p-orbital.
63. Determine the correctness or otherwise of the following Assertion [A] and the Reason [R].
Assertion [A] Water at 100°C and 1 atm is acidic with a pH less than 7.
Reason [R] The ionic product of water Kw decreases, when T increases because the enthalpy of the dissociation of water is endothermic.
(a) Both [A] and [R] are true and [R] is the correct reason for [A]
(b) [A] is false but [R] is true
(c) Both [A] and [R] are true but [R] is not the correct reason for [A]
(d) Both [A] and [R] are false
Ans. (d)
Sol. Assertion : Water at 100°C and 1 atm is neutral with a pH equals to 7.
Reason : The ionic product of water, Kw, increases when T increases because the enthalpy of the dissolication of water is endothermic.
In endothermic reaction, on increasing the temperature, reaction shifts to forward direction.
64. A mixture initially containing 2 mol of CO and 2 mol of H2 comes to equilibrium with methanol, CH3OH, as the product of the reaction CO(g) + 2H2(g) 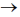 C H3OH(g). At equilibrium, the mixture will contain
C H3OH(g). At equilibrium, the mixture will contain
(a) 2 mol of methanol
(b) more than 1 mol but less than 2 mol of methanol
(c) 1 mol of methanol
(d) less than 1 mol of methanol
Ans. (c)
Sol. 2CO(g) + 2Hg(g) 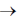 CH3OH(g) + CO
CH3OH(g) + CO
Initially 2 moles 2 moles excess
Here, hydrogen acts as a limiting reagent. When 1 mole of Co(g) react with 2 moles of H2(g). It will form 1 mole of methanol. We have excess of carbon monoxide but limited H2(g). So, reaction will give only 1 mol of methanol.
65. Given that the standard electrode potentials E°(Cu2+/Cu) = +0.340 V and E° (Cu+/Cu) = + 0.522V, the E°(Cu2+/Cu+) is
(a) + 0.862
(b) + 0.182
(c) + 0.158/
(d) – 0.158
Ans. (c)
Sol. 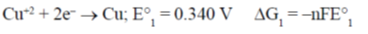
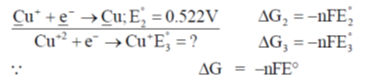
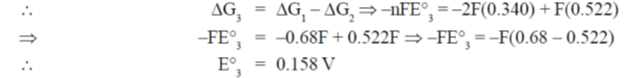
66. The number of water molecules requried to balance the chemical reaction when MnO4– is converted of MnO2 in basic solution is
(a) 1
(b) 2
(c) 3
(d) 4
Ans. (b)
Sol. KMnO4 or MnO–4 is powerful oxidant and probably the most widely used volumetric oxidising agent. It is readily available and colour of its solution is too intense that an indicatoris not required hence, MnO–4 ion act as self indicator.
MnO–4 + 4H+ + 4OH– + 3e–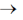 MnO2 + 2H2O + 4OH–
MnO2 + 2H2O + 4OH–
67. For a reaction aA + bB 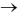 cC + dD, the relation that hold is
cC + dD, the relation that hold is
(a) 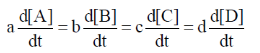
(b) 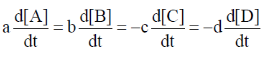
(c) 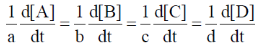
(d) 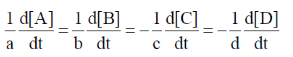
Ans. (d)
Sol. aA + bB 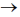 cC = dD
cC = dD
Rate of reaction
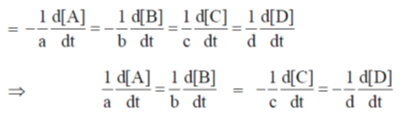
(–) ve sign signifies the decrease in concentration with time.
68. Match the type of transition in the Column I with the frequency of the electromagnetic radiation in the Column II.
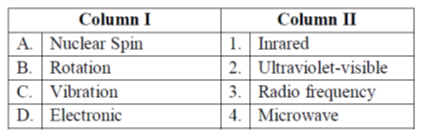
(a) A-1, B-2, C-3, D-4
(b) A-4, B-1, C-3, D-2
(c) A-3, B-4, C-1, D-2
(d) A-3, B-1, C-4, D-2
Ans. (c)
Sol. Nucelar spin : Radio frequency
Rotation : Microwave
Vibration : Infrared
Electronic : Ultraviolet-visible
Therefore, the correct match is
1-C, 2-D, 3-A, 4-B
69. The postulates of Bohr's theroy of the atom are
I. Electrons move in stable circular orbits around the nucleus
II. Electrons may absorb light of specific energy and the be excited to higher energy states
III. Angular momentum of electrons in stable orbits is quantised
IV. Angular momentum of electrons in stable orbits in uncertain
Choose the correct option.
(a) I, II, III and IV
(b) I and II
(c) I, II, and III
(d) I, II, and IV
Ans. (c)
Sol. According to Bohr's theory
(i) Electron moves in stable circular orbit
(ii) Electron can absorb energy from some source and jump from lower energy orbit to a higher energy orbit i.e.,
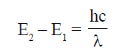
(iii) Angular momentum of electron in stable orbit is quantised i.e.,
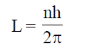
70. Determine the correctness or otherwise of the following Assertion [A] and the Reason [R].
Assertion [A] Blood pressure in humans is greater at the brain than at the feet
Reason [R] Human heart is farther from the feet than the brain
(a) Both [A] and [R] are true and [R] is the correct reason for [A]
(b) [A] is false fut [R] is true
(c) Both [A] and [R] are true but [R] is not the correct reason for [A]
(d) Both [A] and [R] are false
Ans. (b)
Sol. Blood pressure is the measure of the force of blood pushing against blood vessel walls. As human heart is farther from feet when copared to the brain.
So, the blood pressure in human beings is greater in feet than at the brain as the blood has to be pushed back to the heart from feet against the force of gravity and has to travel a large distance from feet to heart. While, in case of brain, the distance is less due to which not much force is required. Thus, the assertion given is wrong and the reason is correct.
71. A jellyfish appearing translucent in the sea disappears, when immersed in a aquarium having liquid X. If the refractive index of the jellyfish is n, refractive index of X is
(a) 1/n
(b) n
(c) 1/2n
(d) 2n
Ans. (b)
Sol. If an object is immersed in a liquid with refractive index same as that of liquid, then it disappears becasue light ray passes undeviated.
72. The dimensions of shear strain are
(a) M0L1T–2
(b) M1L1T–2
(c) M0L1T0
(d) M0L0T0
Ans. (d)
Sol. Shearing strain is given by 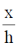
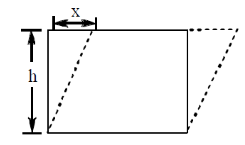
So, it is dimensionless quantity. i.e., (M0L0T0)
73. In SI units, electric flux, which is the dot product of electric field intensity and area vector, are
(a) C/m2N
(b) Cm2/N
(c) Nm2/C
(d) N/m2C
Ans. (c)
Sol. As electric flux (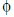 ) is given by
) is given by
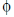 = E . A = |E| |A| cos
= E . A = |E| |A| cos 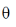
Unit of electric field E = Nc–1
and area, A = m2
So, SI unit of flux 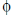 = N m2c–1
= N m2c–1
74. The Zeroth law of thermodynamics
(a) gives a fundamental limitation of the efficiency of a heat engine
(b) deals with thermal equilibrium leading to the concept of temperature
(c) is a direct consequence of the general law of conservation of energy
(d) implies that the coefficient of performance of a refrigerator can never be infinite
Ans. (b)
Sol. Zeroth law of thermodynamics states that if a body A is in thermal equilibrium with body B and body B is in thermal equilibrium with body C, then we can say that body A and C are also in thermal equilibrium.
75. If a human heart beats at an average frequenct of 1.25 Hz, the number of beats per minutes is
(a) 75
(b) 60
(c) 85
(d) 120
Ans. (a)
Sol. To calculate the number of times a heart beat in a minute.
Time taken (t) = 1 min = 60 sec
Number of time it beats = x
Average frequency = 1.25 Hz
Frequency = 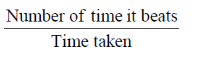
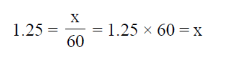
1.25 = = 1.25 × 60 = x
Hence, x = 75 beats/min
76. For a mammalian skeletal muscle, if the extracellular potassium ion concentration,
[K+]out = 4 mM and the intracellular potassium ion concentration, [K+]in = 128 mM, the approximate potassium ion (K+) potential (in mV) is
Assume Faraday's constant = 9.65 × 104 Com–1; Gas constant = 8.31 JK–1 mol–1; Mammalian temperature = 37°C
(a) –47
(b) –94
(c) –27
(d) 0
Ans. (b)
Sol. This problem is based on concentration cell.
For concentration cell E°cell = 0
Now, from Nernst equation
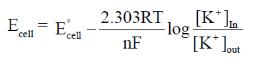
Given, F = 9.65 × 104
R = 8.31 JK–1 mol–1
T = 37° C = 37 + 273 = 310 K
E°cell = 0 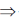 n = 1
n = 1
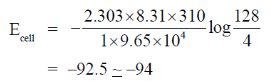
Putting all these values in the above equation we get
77. Optical resolution of light microscope is limited by
(a) size of the specimen being observed
(b) size of specimen stage
(c) intensity of light
(d) wavelength of visible light
Ans. (d)
Sol. For a microscope, optical resolution is given by
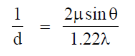
Optical resolution is dependent on refractive index and wavelength of light used.
78. Aqueous environment is a spherical endosome a closed vesicle of 100 nm diameter, is at a pH 5.0 in order to denature and hydrolyse the material internalised by a cell. Assuming Avogadro's number to be 6 × 1023, the number of free protons in an endosome is closest to
(a) 24
(b) 23
(c) 2400
(d) 300
Ans. (b)
Sol. Given diameter = 100 nm

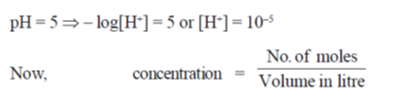
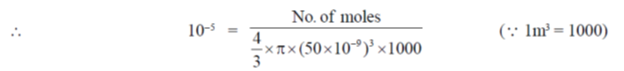
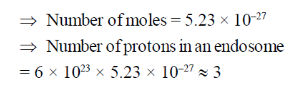
79. Newton's second law of motion deals with
(a) conservation to total mementum of an isolated system of particles
(b) acceleration of a body as a result of applying an external force
(c) rate of change of momentum as a result of applying an external force
(d) the magnitude and direction of forces occurring between pairs of bodies
Ans. (c)
Sol. Newton's second law of motion tells about the rate of change of momentum due to application of external force i.e.,
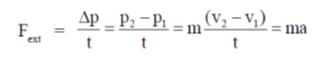
80. If the work done by a human heart is 0.5 J per beat, with the time period 'T' of 0.8 seconds for a beat, the approximate energy (in kcal) required by a human heart to beat in a day is Assume 1 calorie = 4.2 J
(a) 13
(b) 26
(c) 130
(d) 260
Ans. (a)
Sol. Given, work done by a human heart = 0.5 J per beat.
Total number of beat in a day is given by
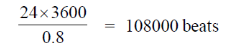
Since, 1 beat requires 0.05 J
So, 108000 beats require = 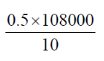
= 54000 J
As we know, 4.2 J = 1 cal
54000 J = 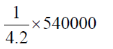
= 12.857 kcal
 13 kcal
13 kcal
81. The maximum depth (in m) rechable by a research submarine, designed to withstand an external pressure of 100 atm, is
Assume Density of water = 1000 kg/m3, accelearation due to gravity = 10m/s2 and atmospheric pressure at the water surface = 1 atjm = 105 Pa
(a) 99
(b) 990
(c) 9900
(d) 99000
Ans. (b)
Sol. As we know, pressure at depth h is given by
p = p0 + 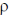 gh
gh
( ρ= density of water, p0 = atmospheric pressure)
ρ= density of water, p0 = atmospheric pressure)
p = 105 + 103 × 10 × h
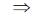 107 = 105 + 104h
107 = 105 + 104h
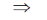 107 – 105 = 104h
107 – 105 = 104h
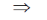 105(100 – 1) = 104h
105(100 – 1) = 104h
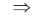 h = 10 × 99 = 990 m
h = 10 × 99 = 990 m
82. The number of water molecules present in a 300 residue soluble protein in spherical shape (diameter = 2 nm) having 20% (V/V) water is closest to
Assume Density of water = 1000 kg/m3, Avogadro's number = 6 × 1023
(a) 224
(b) 9
(c) 140
(d) 28
Ans. (d)
Sol. Volume of spherical shape protein = 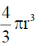
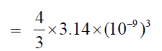
= 4.1867 × 10–27
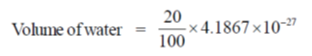
= 0.8373 × 10–27
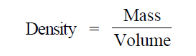
Mass of water = 0.8373 × 10–27 × 103
= 0.8373 × 10–24 kg
= 0.8373 × 10–21 g
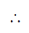 Number of water molecules
Number of water molecules
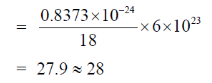
83. If the from bases, A, T, G and C occur with equal likelihood in a five nucleotide DNA sequence, the approximate probability of finding the sequence CGAGT through random chance is
(a) 0.06250
(b) 0.01563
(c) 0.00391
(d) 0.00098
Ans. (d)
Sol. Each nucleotide in a DNA can be filled in four different ways. So, the total number of ways can be 45 = 1024
The number of way of finding CGAGT = 1
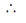 The probability of finding the given sequence CGAGT through random chance is
The probability of finding the given sequence CGAGT through random chance is
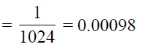
84. A new life form has DNA with 5 nucleotides instead of 4, 5 naturally occurring amino acids instead of 20 and a codon size of 2 bases instead of 3. Assuming the central dogma of biology applies to the new life form , the degeneracy of its genetic code
(a) is likely to be more than ours
(b) is likely to be less than ours
(c) is likely to be identical to ours
(d) does not exist
Ans. (a)
Sol. Degeneracy is known as the redundancy of genetic code means that a single amino acid may be coded by more than one genetic code. Thus, according to the situation given, if such conditions appear in a new life form, the degeneracy of new life form will likely to be more than ours due to such changes in the nucleotide and protein patterns.
85. 20 microbial coloines appear on an agar plate having sucrose and cellujlose as the only carbon sources. Of the 20 colonies, microbes in 12 colonies can metabolise surcose. If microbes in 4 colonies metabolise both sucrose and cellulose, the number of colonies with microbes which can metabolise cellulose is
(a) 4
(b) 8
(c) 12
(d) 20
Ans. (c)
Sol. Total number of microbial coloines appearing on agar plate 'A' = 20
Total number of colonies dependent on sucrose n1 = 12
Total number of colonies dependent on both sucrose and cellulose n2 = 4, n3 = ? (dependent on only cellulose)
'n' = n1 + n3 – n2
20 = 12 + n3 – 4
20 – 4 = 12
n3 = 24 – 12  n3 = 12
n3 = 12
Hence, 12 colonies are dependent on cellulose
86. A heavy chain of an immunoglobulin is a result of recombination of on DNA segment each from 200 different V segments, 12 D segments and 4 J segments from the corresponding DNA sequence. Further, a light chain results from recombination of one segment each from 200 different V segments with 5 different J segments. If a heavy chain and a light chain form an immunoglobulin, the maximum number of different immunoglobulins that can be synthesised is closest to
(a) 103
(b) 105
(c) 107
(d) 109
Ans. (c)
Sol. According to the conditions given for the heavy and light chain of an immunoglobulin molecule.
The different segments consitutes as : Immunoglobulin molecule
= Segments of heavy chains × segments of light chains
Thus, segments of heavy chain are
= 200 × 12 × 4 = 9600
And segments of light chain are
= 200 × 5 = 1000
Hence, 9600 × 1000 = 9600,000 = 107 (approx)
87. Weight of a colony of a newly discovered unicellular organism can be predicted by the empirical equation W (in g) = (x)n, where x = 1.01 and n = number of cells in the colony. If a dog weighs 10 kg, the correct statement amongst the following is
(a) A colony of one million cells is lighter than a dog
(b) A colony of one million cells is heavier than a dog
(c) A colony of one million cells weighs the same as a dog
(d) None of the above
Ans. (b)
Sol. The given empirical equation is
W = (X)n = (1.01)n
where, n is the number of cells in the colony
On considering n = 1,000,000 we get
W = (1.01)1000000 = 1 + (0.01)1000000
= 1000000C0(0.01)° + 1000000C1(0.01)1 + other positive term
[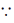 (1 + x)n = nC0x0 + nC1x1 + nC2x2 + ... + nCnxn, where n
(1 + x)n = nC0x0 + nC1x1 + nC2x2 + ... + nCnxn, where n 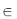 N]
N]
= 1 + 1000000 × (0.01) + other positive term (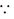 nC0 = 1, nC1 = n)
nC0 = 1, nC1 = n)
= 1 + 1000000 × 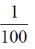 + other positive term
+ other positive term
= 1 + 10000 + other positive term > 10000
Hence, a colony of one million cells is heavier than a dog
88. Given that a heterotrimeric protein is formed by 3 proteins with their centre of masses at the coordinates (1, 1, 2), (3, – 5, 7) and (–1, 7, –6) respectively, the coordinates of the centroid of the triangle formed by joining the three centre of masses is
(a) (3, 3, 3)
(b) (3, 1, 3)
(c) (1, 3, 1)
(d) (1, 1, 1)
Ans. (d)
Sol. Coordinates of the centroid of the triangle formed by joining the three centre of masses are
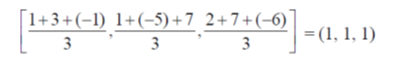
89. Two linear and parallel RNA strands, defined by the equations 3x – 4y + 6 = 0 and 3x – 4y + 5 = 0 are hydrogen bonded together the two strands is
(a) 0.2
(b) 1.0
(c) 1.2
(d) 2
Ans. (a)
Sol. We know that, distance between two linear and parallel strands Ax + By + C1 = 0 and Ax + By + C2 = 0 is
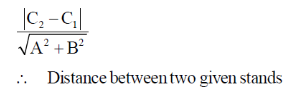
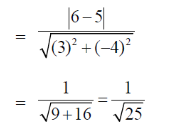
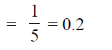
90. (p, q + r), (q, r + p) and (r, p + q) are the coordinates of 3 co-planar atoms in a molecular structure. Area occupied by the three atoms is
(a) pq + qr + pr
(b) pqr
(c) p2 + q2 + r2
(d) None of these
Ans. (d)
Sol. t is given that, (p, q, r), (q, r + p) and (r, p + q) are the coordinates of 3 co-planar atoms.
Then, the area occupied by three atoms is
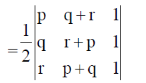
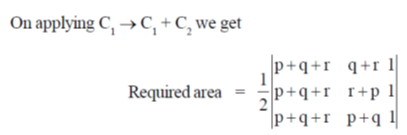
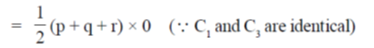
= 0
91. The angle between two linear transmembrane domains defined by the following vectors is
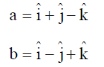
(a) cos–1 (–1/3)
(b) cos–1(1/3)
(c) sin–1(–1/3)
(d) sin–1(1/3)
Ans. (a)
Sol. Let 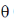 be the angle between two linear transmembrane domains. Then,
be the angle between two linear transmembrane domains. Then,
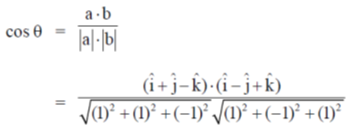
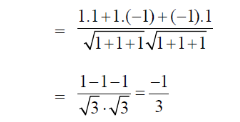
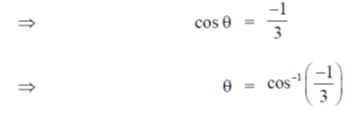
92. A straight DNA segment defined by 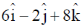 is bound to a linear transcription factor defined by
is bound to a linear transcription factor defined by 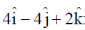 . The correct statement amongst the following is
. The correct statement amongst the following is
(a) The transcription factor is parallel to the DNA segment
(b) The transcription factor is perpendicular to the DNA segment
(c) The transcription factor is at an angle of 45° from the DNA segment
(d) None of the above
Ans. (b)
Sol. Given, DNA segments are
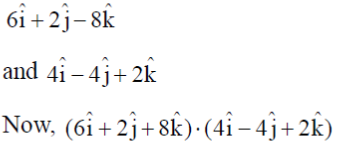
= (24 – 8 – 16) = 0
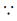 Scalar product of DNA segments is zero
Scalar product of DNA segments is zero
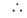 Transcription factor is perpendicular to DNA segment.
Transcription factor is perpendicular to DNA segment.
93. A protein backbone can be traced by plotting the coordinates of 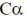 atoms of its constituent amino acids. If area of the triangle formed by joining the coordinates of
atoms of its constituent amino acids. If area of the triangle formed by joining the coordinates of 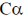 atoms of a tri-peptide is zero,
atoms of a tri-peptide is zero,
(a) the tri-peptide is linear
(b) the tri-peptide is circular
(c) the tri-peptide forms a curved loop
(d) the tri-peptide forms an open curve
Ans. (a)
Sol. If area of the triangle is zero, then the coordinates of the points is vertices will be co-linear. Therefore, the tri-peptide is linear in structure.
94. Priyanka has blood type O. Her mother's blood type is B and father's blood type is A. If both of Priyanka's grandmother had blood type AB and assuming no child was adopted, blood type of either of her grandfather cannot be
(a) A
(b) B
(c) AB
(d) O
Ans. (c)
Sol. If Priyanka's grandmother has a blood group AB, and that of ther father is A (because children born to her can be either AB, A or B)
Now, if Priyanka's father with blood group A marries her mother with blood group B.
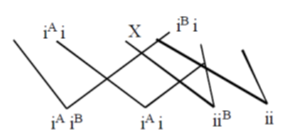
Then, the possibility of children having blood group is either AB, A, B, O. Due to this reason, Priyanka has blood group O. This shows that Priyanka's grandfather can have a blood group A, B and even O excepting AB.
95. Given that you have 2 parents, 4 grandparents, 8 great grandparents, and so on, the number of your ancestors during ten (10) generations of your family preceding you is
(a) 510
(b) 1022
(c) 2046
(d) 2046
Ans. (c)
Sol. Clearly, number of ancestors forms a GP with first term a = 2 and common ratio r = 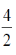 = 2.
= 2.
Now, number of ancestors during ten generations
= Sum of first 10 terms of GP
= S10
= 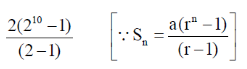
= 2(1024 – 1)
= 2(1023) = 2046
Hence, the number of ancestors preceding you is 2046.
96. The intracellular non-enzymatic fractional degradation of a compound X, f(x) is related to its concentration x through
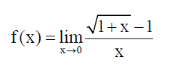
At a negligible concentration of X, its fractional degradation is
(a) 0.00
(b) 0.25
(c) 0.50
(d) 0.75
Ans. (c)
Sol. We have,
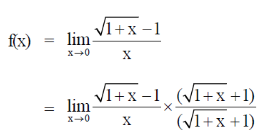
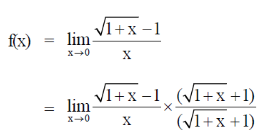
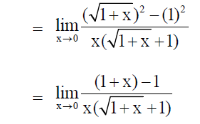
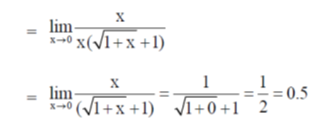
Thus, at a negligible concentration of X, its fraction degradation is 0.50.
97. The distance x 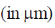 covered by a molecular starting from point A at time t = 0 and stopping at another point B is given by the equation x =
covered by a molecular starting from point A at time t = 0 and stopping at another point B is given by the equation x = 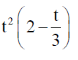
The distance between A and B (in µm) is closest to
(a) 10.7
(b) 20.7
(c) 40.7
(d) 50.7
Ans. (a)
Sol. Given, distance equation is
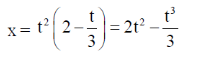
To find the minimum distance, calculate 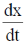 and substitude it equal to zero.
and substitude it equal to zero.

On substituting 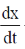 = 0, we get
= 0, we get
4t – t2 = 0
 t(4 – t) = 0
t(4 – t) = 0
 t = 0 or t = 4
t = 0 or t = 4
Hence, the distance between A and B is minimum wjhen t = 4 and the minimum distance is
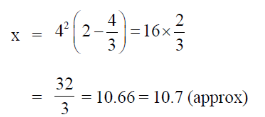
98. Dependence of the weight, y (in kg), of an organism on the number of hours, x when it is in motion, is given by the differential equation 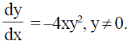
Given that y = 1, when x = 0, the weight of the organism after moving for one hour is
(a) 0.11
(b) 0.33
(c) 0.67
(d) 0.75
Ans. (b)
Sol. Given, differential equation is
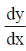 = –4xy2, y ≠ 0
= –4xy2, y ≠ 0
On separating the variables, we get
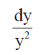 = –4x dx
= –4x dx
On integrating both sides, we get
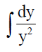 =
= 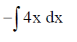
On integrating both sides, we get
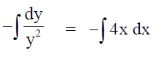
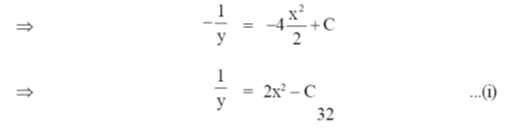
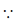 y = 1 when x = 0
y = 1 when x = 0
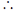 1 = 2(0) – C
1 = 2(0) – C
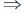 1 = –C
1 = –C
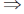 C = –1
C = –1
Now, from Eq. (i), we get
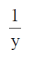 = 2x2 + 1
= 2x2 + 1
Now, when x = 1
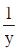 = 2(1)2 + 1 = 2 + 1 = 3
= 2(1)2 + 1 = 2 + 1 = 3
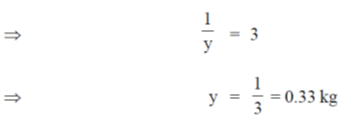
Hence, the weight of the organism after moving for one hour is 0.33 kg.
99. A hospital has 35 patients, 24 of which are HIV+ and 16 have TB infection. All patients have at least one of the two infections. The number of patients with both HIV+ and TB infection is
(a) 5
(b) 8
(c) 9
(d) 11
Ans. (a)
Sol. The total number of patients in hospital are = 35
Number of patients with HIV+ = 24
Number of patients with TB = 16
The nubmer of patients having both
HIV+ and TB = Number of patients with HIV+ + Number of patients with TB – Total number of patients
= 24 + 16 – 35
= 40 – 35 = 5
Hence, the number of patients having both HIV+ and TB are 5.
100. The average weight of four kids is 29.6 kg. If three of the kids weigh 29.8 kg, 28.6 kg and 29.7 kg respectively, the weight of the fourth kid (in kg) is
(a) 29.3
(b) 29.6
(c) 30.3
(d) 30.6
Ans. (c)
Sol. Let the weight of fourth kid is x kg
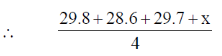 = 29.6
= 29.6
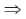 88.1 + x = 118.4
88.1 + x = 118.4
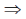 x = 30.3 kg.
x = 30.3 kg.
