IIT JAM Biology 2013
Previous Year Question Paper with Solution.
1. Continuous culture technique at stady state (chemostat) is useful in isolating bacteria from a mixed culture. It exploits the differences in their
(a) maintenance requirements
(b) specific growth rates
(c) rates of specific product formation
(d) endogenous metabolism
Ans. (b)
Sol. Continuous culture is an open system in which at sterile nutrient is added to the bioreactor continuously and an equivalent amount of converted nutrient solution with microbe is withdrwal simultaneously from the system under stady state conditions, the concentration of cell remains constant. Thus, the specific growth rate under steady state is dependent on the dilution rate upto a maximum value equal to 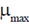 . Therefore, a continous culture may be operated only at dilution rate below the maximum specific growth rate (
. Therefore, a continous culture may be operated only at dilution rate below the maximum specific growth rate (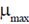 ). If the dilution rate is increase above
). If the dilution rate is increase above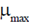 , complete outflow of the cells occurs. Hence, the point at which this condition of prevented is called critical dilution rate (Dcrit).
, complete outflow of the cells occurs. Hence, the point at which this condition of prevented is called critical dilution rate (Dcrit).
2. Biofilm observed in the lungs of cystic fibrosis patients is composed of
(a) polysaccharides
(b) polylactic acid
(c) polypeptides
(d) polyethylene glycol
Ans. (a)
Sol. The persistance of chronic Pseudomonas aeruginosa lung infections in cystic fibrosis (CF) patients is due to a biofilm-growing mucoid (aligninate-producing) strains. A biofilm is structured consoritum of bacteria, embedded in a self-produced polymer matrix consisting of polysaccharide, protein and DNA. In CF lungs, polysaccharide alginate is the major part of the P. aeruginosa biofilm matrix.
3. Match the pathogens listed in Column-I with the diseases listed in Column-II
Column-I Column-II
P. Rotavirus 1. Food poisoning
Q. Leishmania 2. Diarrhea
R. Salmonella Typhimurium 3. Kala azar
S. Epstein-Barr Virus 4. Typhus fever 5. Glandular fever
(a) P–2, Q–1, R–4, S–5
(b) P–2, Q–3, R–1, S–5
(c) P–5, Q–3, R–4, S–2
(d) P–5, Q–1, R–3, S–4
Ans. (a)
Sol. Rotavirus is the most common cause of severe diarrhoea among infants and young children. It is a genus of double-stranded RNA virus in the family Reoviridae. Leishmaina, a genus of trypanosomatid protozoa and is the parasite being responsible for the disease leishmaniasis. It is spread through sandflies of the genus Phleotomus. It is responsible for causeing food poisoning. Salmonella typhimurim is a pathogenic Gram-negative bacteria predominately found in the intestinal lumen. It is responsible for causing typhus or typhoid fever, a symptomatic bacterial infection. Epstein-Barr virus, also known as human herpes viruses, is one of the eight viruses in the herpes family and is one of the most common viruses in humans. It causes glandular fever, a type of viral infection that mostly affects young adults.
4. The time required for an E.coli to divide is less than the time required to replicate its chromosome. This is possible because
(a) of an abundance of enzyymes required for replication
(b) it linearizes the chromosome and replicates from both ends
(c) only one daughter cell receives the nuclear material
(d) of multiple replication forks
Ans. (b)
Sol. Replicatioin of DNA always begins at an origin of replication bidirectionally to the terminus once per division cycle. In bacteria, there is usually one origin per chromosome of plasmid. The cell cycle of slowly growing bacteria is quite similar to that of the eukayotic cells. E.coli is capable of very rapid growth in rich medium, with doubling time as short as 20 min. The replication time however remains long with approximately 60-90 mins. Therefore, the cell cycle is more complicated during rapid growth.
Thus, if the time it takes to synthesise and segregate the daughter chromosomes exceeds one generation time, a new round of replication must be initiated before the previous round is completed. Thus, the cells not replicate with overlapping cycles, but do initiate DNA replication at multiple replication forks on the same copy of the genome.
5. A bacterial population contains a mixture of wild type and leucine auxotrophs. From the mixture, the leucine auxotrophs can be enriched by growing the mixture in minimal medium supplemented with
(a) leucine
(b) pencillin
(c) leucine and penicillin
(d) chloramphenicol
Ans. (c)
Sol. Penicillin enrichment is a parallel technique available for auxotroph selection in bacteria. Many species of bacteria are highly sensitive to the antibiotic penicillin, but only during their proliferating stage. If penicillin is added to a suspension of cells in liquid minimal medium, all the prototrophic cells are killed because thet will cannot divide without supplementation. After treatment, the penicillin can be removed by washing the cells on a filter. Plating the washed cells on a minimum medium supplemented with some specific chemical such as the amino acid leucine.
6. Gram positive bacteria are more rigid than Gram negative bacteria because of the
(a) presence of multiple layers of peptidoglycan
(b) lack of D-amino acids in their cell wall
(c) presence of N-acetygalactosamine in the peptidoglycan backbone
(d) presence of lipopolysaccharide
Ans. (a)
Sol. Gram-positive and Gram-negative bacteria have similar internal, but very different external structures. The cytoplasm of the bacterial cell contains the DNA chromosome, the mRNA, ribosomes, proteins and metabolites. Gram-positive bacterium has a thick of peptidoglycan. The cytoplasmic membranes of most prokaryotes are surrounded by rigid peptidoglycan (murein) layers. The exceptions are Archabacteria and mycoplasmas (have no cell wall at all) because the peptidoglycan provides rigidity and also determines the shape of the particular bacterial cell.
7. Helicobacter pylori can survie in the highly acidic environment of human stomach because it
(a) creates an alkaline microenvironment around itself by urease action
(b) rapidly invades the stomach cells and escapes the acidic environment
(c) is capsulated and hence, is protected from acidic stress
(d) undergoes sporulation for self protection
Ans. (a)
Sol. Helicobacter pylori is an infectious is responsible for peptic ulcer disease marked a turning point in the understanding of gastrointestinal microbial ecology and disease. H. pylori is an S-shaped bacterium 0.5 × 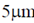 in length. Bacteria began to develop resistance to the strong effects of acid.
in length. Bacteria began to develop resistance to the strong effects of acid.
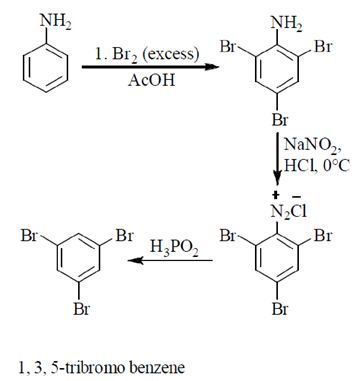
Acid resistance of bacteria such as E.coli and H. pylori, 20 minutes is sufficient for some bacterial cells to reach the mucosa and survival is more likely in the achlorhydic state, at slightly higher pHs. H. pylori bacteria creates an alkaline microenvironment around itself by urease action.
8. The waxy surface of dental plaques does not allow oxygen to diffuse readily. A sample of dental plaque was inoculated into a rich medium and incubated at 37°C with vigorous shaking. However, there was no bacterial growth. The absence of growth was attributed to the following reasons :
P : These bacteria are obligate anaerobes
Q : These bacteria require highly alkaline medium for growth
R : These bacteria require a very hard surface, such as that of teeth, to grow.
Which of these three reasons are likely to be correct?
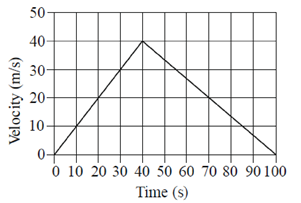
(a) only P
(b) Both P and Q
(c) Both Q and R
(d) Both P and R
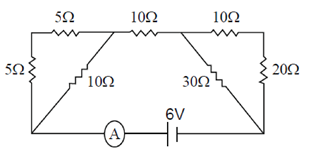
Ans. (d)
Sol. Both statement P and R are correct
Because since waxy surface of dental plaques does not allow oxygen to diffuse readily it allows the growth of bacteria.
These bacteria which are poisoned by oxygen are obiligate anaerobes. Also, they are able to grow only on hard surface like teeth.
Therefore, in an inoculated medium which is not having a hard surface and rich with nutrients and oxygen will not allow bacterial growth.
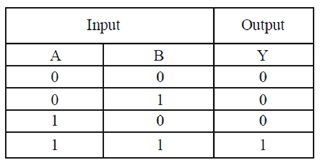
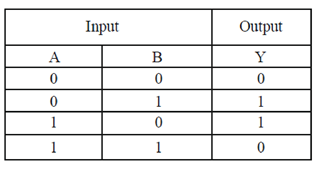
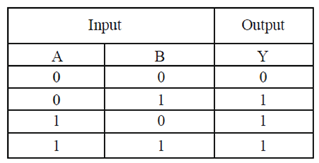
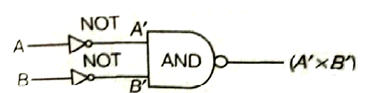
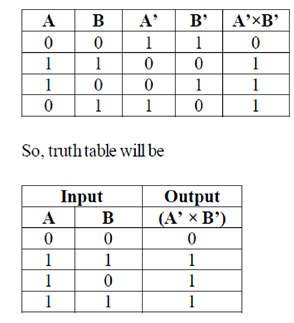
9. Cellulases and hemicellulases are in great demand now a days for the prepartion of second generation
(a) biodiesel
(b) bioplastics
(c) bioethanol
(d) antibiotics
Ans. (c)
Sol. Second generation biofules are made to supply a larger proportion of global fuel sustaninably, affordably and with greater environmental benefits. Thus second generation biofuel like bioethanol processes are addressing is to extract useful feedstocks from woody or fibrous biomass, where the useful sugars are locked in by lignin, hemicellulose and cellulose.
Bioethanol is made by freezing the sugar molecules from cellulose using enzymes, steam heating or other pre-treatments. These sugars can then be fermented to produce bioethanol.
10. Marine microalgae are very important due to their potential industrial significance as single cell coil. These can be used for which of the following?
P: Biofuel production
Q: Recombinant insulin production
R: Atmospheric carbon dioxide sequestration
S: Antibody production
T: Poly-unsaturated fatty acid production
(a) Only P, R and T
(b) Q and S, but not T
(c) Only Q, R and S
(d) P and Q, but not S
Ans. (a)
Sol. Marine microalgae or phytoplankton provide the food base which supports the entire animal population at the open sea.
P. Biofuel production as they have higher photosynthetic efficiency, higher biomas production and higher growth rate besides short life span due to their simple structures compared to other energy crops.
R. Microalgae have a higher CO2 fixation ability compared to plants thus, are potential CO2 sequesters.
T. Refined oils produced by marine microalgae are a potential sources of polyunsaturated fatty acids (PUFA) as they can accumulate lipids in high amounts and to present elevated levels PUFA.
11. Match the enzymes in Column-I with their commercial applications in Column-II.
Column-I
P. Alkaline protease
Q. Asparaginase
R. Immobilized aminoacylase
S. Lipase
T. Immobilized glucose oxidase
Column-II
1. Production of biodiesel
2. Biosensor
3. High fructose corn syrup production
4. Detergent formulation
5. Resolution of DL amino acids and drugs
6. Treatment of lymphocytic leukemia
(a) P–4, Q–6, R–5, S–1, T–2
(b) P–6, Q–5, R–4, S–2, T–1
(c) P–3, Q–1, R–6, S–5, T–4
(d) P–4, Q–5, R–1, S–3, T–6
Ans. (a)
Sol. Alkaline protease are used for cleaning additives in detergents to facilitate the release of proteins. It constitutes to largest product segment in the global industrial enzymes market. They are also used in food, leather, textile, wood, industries.
Asparginase is used as a second or third line treatment of children with Acute Lymphocytic Leukemia (ALL). Three types of asparaginase are currently available, two are derived from E.colil one from Erwinia chrysanthemi.
Immobilised aminoacylase, from Aspergillus oryzae has broad specificity. It is capable of hydrolysing many acyl amino acids.
Lipase, is an enzyme that the body uses to breakdown fats in food, so they can be absorbed in the intestines.
Biodiesel is one of the important biofules and a clean energy source as an alternative to petroleum based diesel fuels.
Lipases are represented as one of the most reproted groups of enzymes for the production of biofuels. They are used for the processing of glycerides and fatty acids for biodiesel production. Immobilised glucose oxidase, is used for the development of a novel glucose biosensor. The glucose biosensor exhibits high selectivity, good repeatability, reproductibility and good stability.
12. A monoclonal antibody (mAb) against a specific cell surface receptor was generated. This was expected to block the receptor function. However, it led to activation instead to blocking. This is due to
(a) cross-linking of the receptors by mAb resulting in receptor activation
(b) binding of the Fab fragment to the cytoplasmic domain of the receptor
(c) binding of the Fc portion to the receptor giving activation signal
(d) the internalization of the mAb by the cell leading to activation
Ans. (a)
Sol. A monoclonal antibody (mAb) aganist a specific cell surface receptor was generated. This was expected to block the receptor function, but instead lead to activation. This happened due to cross linking of the receptors by mAb resulting in receptor activation.
13. Pfu polymerase has
(a) proofreading activity
(b) exonuclease activity
(c) false priming activity
(d) RNA polymerase activity
Ans. (a)
Sol. Pfu DNA polymerase is an enzyme found in hyperthermophillic archaeon Pyrococcus furiosus, where it functions to copy the organisms's DNA during cell division. It is used to amplify DNA in the Polymerase Chain Reaction (PCR) where the enzymes serves the central function of copying a new strand of DNA during each extension step. It has the superior thermostability and proofreading properties compared to Taq polymerase. It possesses 3' to 5' exonuclease proofreading activity.
14. Match the biotech products listed in Column-I wit the applications in Column-II.
Column-I
P. Polyhydroxy alkanoates
Q. Biosurfactants/bioemulsifiers
R. 6-minopenicillanic acid (6-APA)
S. Docosahexanoic acid (DHA)
T. Dextran
Column-II
1. Size exclusion chromatography
2. Nutraceuticals/functional foods
3. Human therapy and diagnostics
4. Bioplastics
5. Precursor for 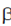 -lactam antibiotics
-lactam antibiotics
6. Microbial enhanced oil recovery
(a) P–2, Q–4, R–6, S–3, T–1
(b) P–4, Q–6, R–5, S–2, T–1
(c) P–5, Q–1, R–4, S–6, T–3
(d) P–4, Q–5, R–1, S–3, T–6
Ans. (b)
Sol. The correct match are as follows :
1. Polyhydroxy alkanoates (PHA) are linear polysters produced in nature by bacterial fomentation of sugar or lipids, and used in making biodegradable plastics.
2. Biosurfactants/bioemulsifiers are successfully used in microbial enhanced oil recovery.
3. 6-APA serves as the intermediate for the formation of all synthetic penicillins and β-lactam antibiotics are all antibiotic agents that contain 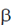 -lactam ring in their molecular structures including penicillin derivates (pernams)
-lactam ring in their molecular structures including penicillin derivates (pernams)
4. DNA is a nutracentical, i.e., omega 3 fatty acid known to be essential lipid component in the eye, brain and nervous tissues. It is helps to improve congnitive functin in humans.
5. Size exclusion chromatography is used to separate molecules by their size and in some cases molecular weight. In this process dextran gels are used.
15. Match the materials listed in Column-I with the techinques/purpose listed in Column-II
Column-I
P. Phenyl agarose
Q. Ni-NTA matrix
R. TEMED chromatography
S. Silicone oil
Column-II
1. Antifoaming agent
2. Polymerase chain reaction
3. Hydrophobic interaction
4. Gel electrophoresis for proteins
5. Affinity chromatography
(a) P–5, Q–1, R–4, S–3
(b) P–4, Q–1, R–2, S–5
(c) P–3, Q–5, R–4, S–1
(d) P–5, Q–1, R–4, S–2
Ans. (c)
Sol. The correct match for the following are
1. Phenyl agrose is used in protein chromatography, affinity chromatography and hydrophobic interactions.
2. Ni-NTA agarose is an affinity chromatography matrix for purifying recombinant proteins carrying a His tag.
3. Tetramethylethylenediamine (TEMED) is an essential catalyst for polyacrylamide gel polymerisat
4. A silicon oil is any liquid polymerised siloxane with organic side chains. Simethicone, i.e., silicon oil is a potent anti-foaming agent due to its low surface tension.
16. Determine the correctness or the wide of the following Assertion [a] and Reason [r] :
Assertion [a] : HeLa cells, derived from cervical cancer tissue and fibroblasts from a normal human male CANNOT divide indefinitely in cell culture.
Reason [r] : Cells undergo sensescence (i.e. ageing) even when grown in cell culture
(a) Both [a] and [r] are true, and [r] is the correct reasong for [a]
(b) Both [a] and [r] are true, but [r] is NOT the correct reason for [a]
(c) [a] is false but [r] is true
(d) Both [a] and [r] are false
Ans. (a)
Sol. A HeLa cell is a cell type in an immortal cell line used in scientific research. It is the oldest and the most commonly used human cell line. The line was derived from cervical cancer.
The cell line was fouhd to be remarkably durable and peofilic, which has led to its contamniation of many other cells lines used in research. The HeLa cell line cannot divide indefinityel but due to mutation have evaded normal cellular sensescence and instead keep undergoing division. Thus, both (A) and (R) given are true and (R) is the correct reason for (A).
17. A human gene was PCR amplified from a cDNA library, cloned into an expression vector and transformed into E.coli. However, the expression of this gene was poor. This is probably because of
(a) the presence of introns
(b) the modifications of the mRNA that are unique to eukaryotes
(c) the incompatibility of the mRNA with E.coli ribosome
(d) a shortage of tRNA for certain codons used in the human gene
Ans. (c)
Sol. E.coli one of the organisms of choice for the production of recombinant proteins. It use as a cell factory, is well-established and has become the most popular expression platform. Obtaining a recombinant protein is preety straight forward. Gene of interest if after transforming into the host of choice is not characterised properly or the expression of it tends to be poor. This particularly means that the mRNA produced is not much compatible with the ribosomes of host (E. coli etc). Hence, the protein can not be ready for purification and characterisation.
18. A student wanted to clone a gene G (size ~1.6kb) in a vector V (size ~ 5kb). The vector has sites for restriction enzymes RE1, RE2 and RE3 in its multiple cloning site. The site for RE2 is located in between the sites for RE1 and RE3. The student carried out the following steps for cloning :
Step 1. Cut the gene G with restriction enzymes RE1 and RE3.
Step 2. Cut the vector V with restriction enzymes RE1 and RE3
Step 3. Ligated G and V from Steps 1 and 2, transformed into E.coli and isolated plasmid L from the transfomants.
The plasmoid L was cut with RE2, which gave a band of size ~ 6.6kb.
The following three reasons were given to explain this observation :
P: Plasmid L is same as the vector V, since E.coli got transformed only with uncut V.
Q: Gene G has a site for RE2.
R: RE1 and RE2 are isochizomers
Which of the above three reasons are possible?
(a) P alone, neither Q nor R
(b) R alone, neither P nor Q
(c) Either Q or R, but not P
(d) Q alone, neither P nor R
Ans. (d)
Sol. Through the experiment given, it is eventually proved that gene G and vector V were cut with the restriction enzymes RE1 and RE3. But, the RE1 and RE3 are not isoschizomers because they are not the pairs of restriction enzymes that are specific to the same recognition sequences.
The isoschizomers are isolated from the different strains of bacteria and therefore, may require different reaction conditions. Instead both RE1 and RE3 are neoschizomer (recognises same sequence but cuts differently). Thus, the only correct option/explanation is that gene G has a site for RE2 restriction enzyme.
19. The following is a schematic of the Iac operon :

Which one of the following statements is correct?
(a) Iacl, being part of the operon, is a structural gene
(b) P, being farthest from IacA, is trans-acting
(c) IacZ is a structural gene and it codes for an enzyme
(d) Iacl is cis-acting, but the protein encoded by it is trans-acting
Ans. (c)
Sol. Lac operon is a operon required for the transport and metaboism of lactose in an E.coli and many other enteric bacteria.
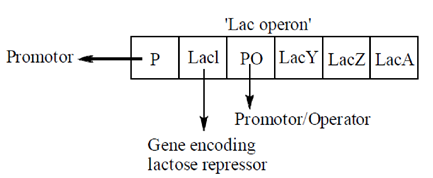
Lac l, is not the part of the operon, produces a protein that blocks RNA from binding to the promoter of the operon. The protein formed by the lacl gene is known as the lac-reprossor. In the context of regulation of transcription, a trans-acting element is usually a DNA sequence that contains a gene coding for a protein being used for the regulation of anther target gene. But, the activity is via the intermediary protein or RNA that it encodes.
Cis-acting elements on the other hand does not code for protein or RNA. But, the trans-acting gene and the protein/RNA it encodes are said to 'act in trans' on the target gene. Thus, this protein encoded by it is cis-acting, Lac-B is trans-acting but the protein encoded by it is cis-acting. Hence, the only correct option is that Lac Z is a structural genes coding for an enzyme 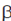 -galactosidase.
-galactosidase.
20. Mammalian cells in the S phase can be monitored by incorporating which one of the following in the cell culture medium?
(a) 35S Methionine
(b) 32P-ATP
(c) 3[H] Thymine
(d) 3[H] Thmidine
Ans. (d)
Sol. In mammalian cells, the synthesis of DNA is confined to a discrete period of the cell cycle called S phase. The rate of DNA synthesis of a population of cells in tissues or cultures depends on the number of cells in S phase cell. Thus, of radioactive nucleotide such as [H] thymidine to measure the rates of DNA synthesis, adds the complications of uptake, pool sites, activities of nucleotide synthesising and turn over.
21. The following experiment was performed with a double stranded DNA sample S of size 1 kb :
Step 1 : The two strand were separated
Step 2 : The separtated strands were anealed
Step 3 : Treated the double stranded DNA from Step 2 with 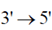 exonuclease
exonuclease
From agarose gel electrophoresis, it was found that the DNA from Step 3 is less than 1 kb. The most probable reasong for not getting a 1kb DNA in Step 3 is that the DNA in sample S
(a) is palindromic
(b) has sequence repeats
(c) has sequence inversion
(d) has strands, each of which has self-complementary sequence
Ans. (d)
Sol. In the given experiment after agrose gel electrophoresis, it was found that the DNA from step 3 is less than 1 kb. This may be due to the fact that the double stranded DNA sample must be having strands, each of which has self-complementary sequence.
Self complementary refers to the fact that a sequence of DNA may fold back on itself, creating a double strand like structure. This decreases the size of the sample.
22. A batch of students studied the effect of changing either phospholipid composition or temperature on the transport of solutes across liposomal membranes. From these studies, the students arrived at the following conclusions :
P : Increase in the saturation of hydrogen chain led to an increase in the rate of diffusion of oxygen
Q : Decreasing the temperature to 5°C led to a decrease in the rate of potassium transport by valinomycin (a carrier ionophore)
R : Increase in the hydrocarbon chain length did not have any effect on the transport mediated by gramicidin (a channel ionophore)
Which of these conclusion (s) is/are NOT correct?
(a) Only R
(b) Only Q and R
(c) Only P
(d) Only P and Q
Ans. (c)
Sol. Statement P is incorrect. As on studying the effect of changing either phospholipid composition or temperature on the transport of solutes across liposomal membranes. It was studied that on increasing order of hydrocarbon chains in saturated and unsaturated membranes, there was significant decrease in the oxygen diffusion concentration product in the hydrocarbon region of these membrane.
23. Circular DNA of a bacterium was allowed to replicate once. In this expermient, some of the nucleotides were fluoroescently labeled. The primase was able to accept the labeled as well as the unlabeled nucleotides. However, the DNA polymerase could use ony the unlabelled nucleotides.
The fluorescent label is found in
(a) both the template and newly made strands
(b) only the newly made strand
(c) only the template strand
(d) neither of the two strands
Ans. (b)
Sol. In the given experiment fluorescent label is found in neither of the two strands. This is because the DNA polymerase could only use the unlabeled nucleotide, and DNA polymerases are enzymes that create DNA moelcules by assembling nucleotides, the building blocks of DNA. Therefore none of te strands will be fluorescent labelled.
24. Consider the following metabolic pathway found in bacteria :
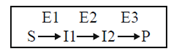
E1, E2 and E3 are enzymes which catalyze different steps of the pathways is indicated above. There are three classes of mutants :
Class I - has defective E1, hence requires |1 in the medium to grow
Class II - has defective E2, hence requires |2 in the medium to grow
Class III - has defective E3, hence requires P in the medium to grow
Wild type grows in the minimal medium
We mix Class I, II and III mutants and allow this mixture to grow in minimal medium.
Suppose we observe growth because of revertants, then reversion has to be in
(a) All three classes
(b) Only class I
(c) Only Class III
(d) Any of the three classes
Ans. (b)
Sol. The growth is observed in minimal medium of culture of class I, II and III mutants. This was due to revertants (a mutant that has reverted to its former genotype or to the original phenotype by means of a suppressor mutation or else by compensatory mutation somewhere in the gene that is second site reversion). The reversion was in class I only.
25. The stain DAPI (4', 6-diamido-2-pheylindole) is used for total cell count because it reacts with
(a) Lipid bilayer
(b) RNA
(c) Membrane proteins
(d) DNA
Ans. (d)
Sol. DAPI (4', 6 diamidino-2-phenylindole) is a fluorescent stain that binds strongly to the A-T rich regions in DNA. It is used extensively in fluorescence microscopy. As DAPI can pass through an intact cell membrane, iot can be used to stain both live and fixed cells though it passes through the membrane less efficiently in the live. The investigation of DAPI indicates that it bound strongly to DNA and became more fluorescent when bound and has a maximum absorption at a wavelength of 358 nm. As it is a DNA binding compound, it is likely to have some low level mutagenic properties and care should be taken in its handing and disposal.
26. Which one of the following statements about cytoskeletal proteins is NOT TRUE?
(a) They give shape to the cell
(b) They are non-essentialproteins
(c) They help in cell division
(d) They help in cell motility
Ans. (b)
Sol. Cytoskeleton is an intracellular matrix that supports the shape and function of the cell. The cytoskeletal proteins are proteins that makes up the cytoskeleton, cilia and flagella of cells. Generally cytoskeletal proteins are polymers, including tubulin actin and lamin. They are known to be essential proteins meant to provide shape, motility and division of the cell.
27. DNA was isolated from three samples of normal human cells. In each of these samples, cells were at different stages of cell division
Sample 1: Cells were in G2 phase
Sample 2: Cells were in anaphase
Sample 3: Cells were in telophase
DNA isolated from which of these samples conforms to Chargaff's rules?
(a) Only samples 1 and 2
(b) Only samples 1 and 3
(c) Only samples 2 and 3
(d) Samples 1, 2 and 3
Ans. (d)
Sol. Erwin Charagaff in mid 1900S found that concentration of the four bases different from one species to another.
However within each species, the concentrations of thymine. The same was true of the concentration of guanine and cytosine. In the given question the three samples are G1 phase, anaphase and telophase of cell cycle respectively and in any of the sample Charagaff's rule can be conformed as all three are human cell samples.
28. Which of the following feature are shaped by mitochondria and chloroplasts?
P : The enrgy transducing membrane is highly invaginated
Q : Storma of chloroplast and matrix of mitochondria are alkaline
R : Their number per cell is variable
(a) Only P and Q
(b) Only P and R
(c) Only Q and R
(d) P, Q and R
Ans. (d)
Sol. The membrane found organelles, i.e. both mitochondria and chloroplast are highly organised structures. The mitochondria is structurally defined in large measure by its two membranes, a limiting outer membrane that enwraps the energy transducing inner membrane, on the other hand thylaloids membrane in thechloroplast is also highly invaginated. Both stroma of chloroplast is alkaline, aqeous fulid which is protein rich and is present within the inner membrane of the chloroplast, Similarly, matrix of mitochondria is also alkaline and negatively charged with a series of specific channels and carrier proteins. Also, the numbers of both mitochondria are variables.
29. Cilia and flagella are locomotor appendages found on some cells. A flagellum has an undulatory (i.e., snake-like) motion. A cilium has a back-and-forth (i.e., rocking chair-like) motion. The following statements relate the direction of movement of a cell to the axis of flagellum/cilium that drives its motion :
P : The cell moves along a direction perpendicular to the axis of the flagellum
Q : The cell moves along a direction parallel to the axis of the cilium
(a) Both P and Q are correct
(b) Only P is correct
(c) Neither P nor Q is correct
(d) Only Q is correct
Ans. (c)
Sol. Both cillia and flagella ranges in length from a few microns to more than 2 mm. Although cilia and flagella are the same, they were given different names before their structrues were studied. The beating pattern of both cilia and flagella is different. Flagellum usually undlates, its snake-like motion driving a cell in the same direction as the axis of the flagellum, e.g. propulsion of human sperm cell. On the other hand, cilia have a back-and-forth motion that moves the cell in a direction perpendicualr to the axis of the cilium, e.g. movement shown by a freshwater protozoan.
30. Listed below are some classes of enzymes :
P : Exoproteases
Q : Glycosidases
R : Glycosltransferase
S : Lipid tranferases
Which of the above are associated with post-translational modification of proteins?
(a) All four classes
(b) Only Q, R and S
(c) Only Q and R
(d) Only R and S
Ans. (d)
Sol. Proteins are synthesised by ribosomes translating mRNA into polypeptide chains, which may undergo PTM to form the mature protein product. It plays a key role in many cellular processes such as cellular differentiation, protein degradation, signaling regulatory processes, regulatiion of gene expression and protein-protein interactions. Glyxosyl transferases and lipid transferases are the enzymes being involved in the post translational modification of proteins. Glycosyl transferase is important for transferring the sugar moeity onto proteins. It regulates the protein activity by PTM. On the other hand lipid transferase is known as when transferases uses lipids as an acceptor forming glycolipid to regulate PTM.
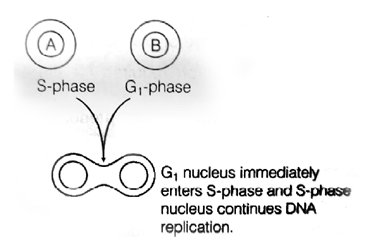
31. Cell A is in S phae of cell divison. Cell B is in G1 phase of cell divison. If cell A and cell B were to be fused then the nucleus of the cell B in the heterokaryon
(a) continues to be in the G1 phase
(b) enters the S phase
(c) enters the M phase
(d) enters the G2 phase
Ans. (b)
Sol. A typical eukaryotic cell cycle is illustrated by human cells in culture, which divides approximately every 24 hrs. 95% of the cell cycle is spent in tnerphase (i.e., period between mitosis).
During this phase, the chromosomes becomes decondensed and distributed throughout the nucleus, so that the nucleus appears morphologically uniform.
At molecular level, interphae is the time during which both cell growth and DNA replicatin occurs in an ordely manner in preparation for cell division.
It comprises of three phases.
1. G1 phase Initiation of DNA replication takes place, cell become metabolically active and continues to grow but does not replicate its DNA.
2. S phase DNA replication actually takes place.
3. G2 phase Cell growth continues and proteins are synthesised in preparation for mitosis.
According to the experiment given, if the nucleus of the cell in G1 phase is allowed to fused with the nucleus of thecel in S phase, the cell in G1 phase is stimulated to proceed into S phase as the components found in G1 phase still has the achieve what will happen further in the S phase, on the contrary, the components in the S phase will continue to replicate as usual, i.e., no change will be observed in the nucleus of the cell of the G1 phase.
32. For a single-enzyme-substrate reaction, the half-life of the enzyme can be calculated using the following expression, where in k is the first order rate constant or deactivation constant
(a) 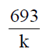
(b) 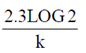
(c) 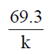
(d) 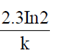
Ans. (b)
Sol. For first order reaction
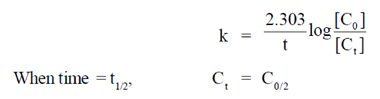
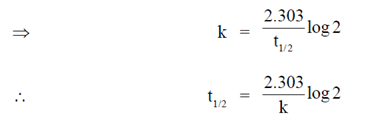
33. The transmittance of a solution containing 10–5 M ATP is 0.75 (75%) at 260 nm in a 1 cm path length cuvette. According to Beer-Lambert law the absorbance is
(a) 0.750
(b) 0.155
(c) 0.125
(d) 0.250
Ans. (c)
Sol. Here, transmittance (T) = 0.75 at 260 nm
According to Beer-Lambert Law,
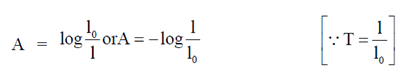
 A = – log T
A = – log T
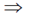 A = – log (0.75)
A = – log (0.75)
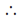 A = 0.125
A = 0.125
34. Which one of the following is applicable to an enzyme catalyzed reaction that follows zero order kinetics as per the Michaelis-Menten model?
(a) 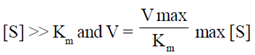
(b) 
(c) 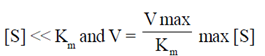
(d) [S] >> Km and V = V max
Ans. (d)
Sol. According to Michaelis-Menten mechanism, enzyme-substrate is formed in the first step and either the substrate unchanged or after modification form products.
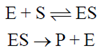
For a given [E]0 and high value of [S], the rate of product formation becomes independent of [S] reaching a maximum value known as the maximum velocity, Vmax.
V = 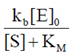
When [S] > > KM the rate reaches its maximum value and is independent of [S]
v = vmax = Kb [E]0
35. The free energy change, under standard conditions, upon hydrolysis is a "high-energy bond" is large and negative. ATP, phosphocreatine, phosphoenolpyruvate and acetyl coenzymes A are examples of molecules that contain such "high-energy bonds". Which of the following are high-energy bonds present in these molecules?
P : N–P
Q : C–S
R : P–O
(a) Only R
(b) Only P and R
(c) Only P and Q
(d) P, Q and R
Ans. (d)
Sol. Certain compounds on hydrolysis yields high energy like phosphoenolpyruvate (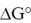 = –14.8), ATP (
= –14.8), ATP (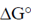 = –7.3), Phosphocreatine (
= –7.3), Phosphocreatine (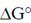 = –10.3), Acetyl CoA (
= –10.3), Acetyl CoA (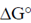 = – 7.7), etc.
= – 7.7), etc.
These contain high energy bonds like N—P, C—S and P—O which on hydrolysis releases large amount of energy.
36. Secretory IgA (sIgA) can exist in a protease rich mucosal environment because
(a) the secretory component masks the sites suceptible to proteases
(b) the action of proteases in neutralized by sIgA
(c) sIgA is secreted in vesicles and hence protected from proteases
(d) the J chain masks the protease-susceptible sites
Ans. (d)
Sol. Although IgA constitutes only 10-15% of the total immunogloin in serum, it is the predominant immunoglobulin class in external secretions such as breast milk, saliva, tears and mucus of the branchial, genitourinary and digestive tracts.
The plasma cells that produces IgA preferentially migrates to sub-epithelial tissue, where the secreted IgA binds tightly to the receptor for producign polymeric immunogloulin molecules. This poly-lg-receptor is expressed on the basolateral surface of most mucosal epithelia and on glandular epithelia. After the binding of polymeric IgA to the poly-lg receptor, the receptor-IgA complex is transported across the epithelial barrier to the lumen.
Transport of receptor IgA complex involves receptor-mediated endocytosis and directed transport of the the vesicle across the epithelial cell to the luminal membrane. for the fusion of vesicles is then cleaved enzymatically from the membrane and becomes the secretory component, that is bound to and released together with a polymeric IgA into the muscus secretions.
Hence, the secretory component, masks the sites susceptible to protease cleavage in the hinge region of secretory IgA, allowing the polymeric molecules exist longer in the protease-rich mucosalenvironment interacting with the J-chain of both polymeric IgA antibody then would be possible otherwise.
37. Suppose that the surface area of an erythrocyte is 167 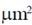 . Also, suppose that the membrane of this erythrocyte contains 4.15 × 10–16 mol of phospholipid and 3.1 × 10–16 mole of cholesterol.
. Also, suppose that the membrane of this erythrocyte contains 4.15 × 10–16 mol of phospholipid and 3.1 × 10–16 mole of cholesterol.
The cross-sectional area of a phospholipid molecule is 0.7 nm2 and that of a cholesterol molecule is 0.38 nm2. Phospholipid and cholesterol isolated from this membrane were spread into a monolayer. The ratio of the area of this monolayer to that of theerythrocyte is approximately
(a) 0.5
(b) 1.0
(c) 1.5
(d) 2.0
Ans. (d)
Sol. Area of phospholipid molecule
= 4.15 × 10–16 × 0.7 = 2.905 × 10–16 nm2
Area of cholestrol = 3.1 × 10–16 × 0.38
= 1.178 × 10–16 nm2
Area of monolayer = 4.083 × 10–16 nm2
Area of erythrocyte = 167 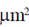 = 167 × 106 nm2
= 167 × 106 nm2
Required ratio = 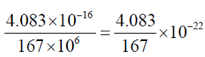 = 2 approx
= 2 approx
38. For the Na+- mediated active transport of a molecule of glutamic acid from a concentration of 0.1mM outside the cell to 20 mM inside the cell, calculate the minimum number of Na+ ions required to supply the necessary free energy.
Use T = 37°C, pH = 7, 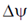 = –70mV and F = 23062 cal/mol. V.
= –70mV and F = 23062 cal/mol. V.
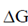 released for the transport of one Na+ is 3.3 kcal/mol. Glutamic acid carries a net charge of –1 at pH 7.
released for the transport of one Na+ is 3.3 kcal/mol. Glutamic acid carries a net charge of –1 at pH 7.
(a) 4
(b) 10
(c) 2
(d) 1
Ans. (c)
Sol. This problem is based on concentration all for concentration all E° = 0
Now, from Nernst equation
E = E° – 2.303 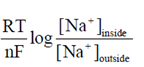 ...(i)
...(i)
Given, F = 23.62 cal/mol V
T = 37°C = 37 + 273 = 310 K
E = 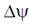 = – 70mV, R = 2 cal/mol, [Na+]inside = 20mM
= – 70mV, R = 2 cal/mol, [Na+]inside = 20mM
[Na+]outside = 0.1 mM
Putting all these values in Eq. (i), we get
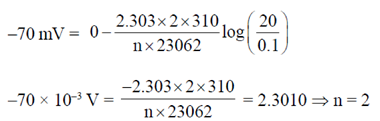
39. NAD and NADP are two important cellular electron carriers. NAD is generally associated with catabolic reactions and NADP with anabolic reactions. A cell will have a fixed number of NAD molecules and a fixed number of NADP molecules. In a normal cell most of the
(a) NAD and NADP molecules will be in the reduced state
(b) NAD molecules will be in the reduced state whereas most of the NADP molecules will be in the oxidized state
(c) NADP molecules will be in the reduced state whereas most of the NAD molecules will be in the oxidized state
(d) NAD and NADP molecules will be in the oxidized state
Ans. (d)
Sol. The cellular rfespiration processes of all living cells makes use of the coenzyme Nicotinamide adenine dinucleotide (NAD) which play a key role in the energy metabolism by accepting and donating electrons. NAD and NADP are one of the most important cofactors found in a normal eukaryotic cell.
Healthy bodies makes all the NADH they need using Vit-B3 as a starting point. NAD coenzyme acts as a hydrogen acceptor in oxidation-reduction reaction. NAD+ in the body acts as an intermediary, oxidising the food moleucles in a much controlled fashion. It is important in its oxidised form in the body because by using NAD+ and the electron transport chain, the oxidation of organic molecule can be linked to the production of ATP rather than just the generation of heat.
40. An animal's body plan has evolved over millions of years to suit its functions.
Given below are a few types of epithelia that line various organ systems
P : Simple squamous epithelia that are thin and leaky
Q : Stratified squamous epithelia that can regenerate rapidly
R : Pseudostratified ciliated columnar epithelia that formes a mucous membrane
S : Cuboidal epithelia specialized for secretion
Which one of the following four options is correct?
(a) The digestive system has Q, but not S
(b) The respiratory system has Q and R, bu not S
(c) The circulatory system has P as well as R
(d) Both excertory and endocrine systems have S
Ans. (a)
Sol. Epithelial tissue is a sheeot of cells that covers a body surface or lines a body cavity. Stratified squamous epithelium is the most widespread epithelia been composed of several layers and is perfect for its protective role. Its apical cells are squamous and cells of deeper layer are either cuboidal or columnar. It is quite thick found continuing with the lining of the digestive system extending every body opening that continues with the skin. Epithelium have a high regenerative capacity when compared to other tissues of the body. Due to which it reproduces rapidly as long as they receive adequate nutrition.
41. Which one of the following four statements is TRUE regarding nitrogen fixation by bacteria?
(a) Nitrogen fixation is primarily an oxidation process requireing continuous supply of electron acceptors
(b) Molybdenum accepts, but cannot donate, electrons within nitrogenase
(c) Symbiotic nitrogen fixers have a respiratory mechanism for protecting nitrogenase
(d) Nitrogen fixation is inhibited in some bacteria due to depletion of carbon source
Ans. (a)
Sol. Nitrogen fixation is a process by which nitrogen (N2) in the atmosphere is converted into ammonia (NH3). The major requirements of nitrogen fixation includes ATP and an electron source.
ATP could be supplied to the process through photophosphorylation, oxidative phosphorylation and by the breakdown of polyphosphates which are abundant in cyanobacteria.
It is a reductive process requiring a continuous source of strong reducing agent. The origin and nature of electron donors used in nitorgen fixation vary among the different physiological group of nitrogen fixers.
In aerobic or symbiotic nitrogen fixation NADH or NADPH acts as the major electron donors. Hence, is an oxidation process that eventually requires a continous supply of electron acceptors.
42. The disciplines of evolutionary biology and ecology are closely linked to each other. The environment plays a major role in determining the prevailing flora and fauna.
Given below are four statements showing this relationship
P : Malaria is quite prevalent in African arid zones
Q : Plants with spiny leaves are commonly found in the tropical rainforests of Borneo
R : Halophiles are more prevalent at the bottom, as compared to the surface, of an estuary
S : Lions are rarely found in African grassland savanna
Which one of the following four options is correct
(a) R is true, but P is false
(b) R and S are true, but Q is false
(c) P and Q are true, but S is false
(d) P and R are false
Ans. (b)
Sol. The environment plays a major role in determining the prevailing flora and fauna. This can be proved by two ways.
1. Haleophiles are the organisms that thrives in high salt concentration or needs salt in their environment to live. They live in evaporation ponds or salt lakes depending upon the type of saline environment in which they flourish, they may be classified as slightly, moderately or extremely halophilic. These organisms live closer to the bottom of an estuary for two general reasons.
(a) Due to denser salt concentration, which therefore increases as a function of depth.
(b) As they mostly tend to be anaerboic, due to which they shy away from oxygenated areas (such as the surface of a water source).
2. The African Savanna grasslands are expansive areas with scattered trees lying between the continent rainforests and deserts that runs along the equator. African Lions (Panthera leo) are the only cats known to live in groups known as prides. Their average life span in the wild is 15-18 yrs and 25-30 yrs in captivity. These are the rarest animals found in African savanna's when compared to other animals such as Elephant, Cheetah, Giraffe, Flemingo, Zebra, Black rhinoceros etc.
43. The pedigree of a family affected by a certain genetic disorder is shown below.
Microsatellite markers from these individuals were PCR amplified, separated by gel electrophoresis and detedted by two probes P1 and P2. Probe P1 is for marker M1 and probe P2 is for marker M2. The resulting band patterns are shown along the with the pedigree diagram.
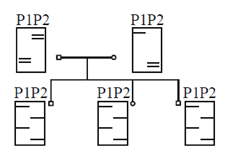
From this data, it can be concluded that the
(a) father is homozygous at both the markers
(b) mother is heterozygous at both the markers
(c) trait is linked only to the marker M1
(d) trait is linked to both markers
Ans. (c)
Sol. In the given pedigree of a family affected by a certain genetic disorder, the resulting band patterns show that the trait is linked only to the marker M1. As the affected individual probe P1 marker M1 one band is present in both affected progeny and another band is present in normal progeny.
44. Which of the following statements is NOT CORRECT?
(a) SDS-PAGE separates proteins based on molecular weight
(b) Gel filtration chromatography can be used to determine the molecular weight of a protein
(c) Ion exchange chromatogaphy separates protein based on their size
(d) Membrane dialysis is commony used for desalting
Ans. (c)
Sol. Ion exchange chromatography is applicable for the separation of charged molecules. It is based on the reversible exchange of ions in solution with ions electrostatically bond to support media (ion exchangers).
45. The radius of first "Bohr's stationary orbit" for hydrogen atom is 0.53Å. The radius of the second orbit is
(a) 1.06Å
(b) 1.59Å
(c) 2.12Å
(d) 4.24Å
Ans. (c)
Sol. Bohr radius (a0) = 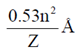
[n = number of orbit = atomic number]
For second orbit, n = 2
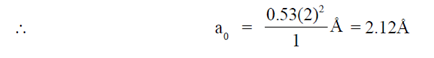
46. Calculate the wavelength of the alpha particle, if its mass = 6.6 × 10–27 kg and velocity = 2 × 107m/s. Assume Planck's constant h = 6.6 × 10–34Js.
(a) 5.0 × 10–12 m
(b) 5.0 × 10–13 m
(c) 5.0 × 10–14 m
(d) 5.0 × 10–15 m
Ans. (d)
Sol. Mass, m = 6.6 × 10–27 kg
Velocity, v = 2 × 107 m/s
According to de-Broglie relation
[symbols have their usual meaning]

= 5 × 10–15 m
47. Among Mg2+, Al3+, F– and O2–, the ions with the lowest and highest ionic radii, respectively, are
(a) Al3+ and O2–
(b) Mg2+ and Al3+
(c) F– and Al3+
(d) F– and O2–
Ans. (a)
Sol. Among Mg2+, Al3+, F– and O2–
Al3+ ion has lowest ionic radii. Al3+ ion formed by the removal of three eklectrons from Al. So, electrons in outermost orbits are less and positive charge of nucleus is increases so, effective nuclear charge increases and outermost orbit is attracted by nucleus so, ionic size decreases. While O2– has highest ionic radii because electrons of outermost shell increases and positive charge of nucleus is decrease and thus effective nuclear charge decreases. Therefore, ionic size increases.
48. The molecule that shows paramagnetism is
(a) F2
(b) N2
(c) B2
(d) C2
Ans. (c)
Sol.
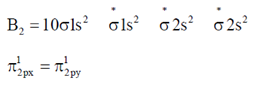
Here, two unpaired electrons are present at π-bonding orbital so, B2 is paramagnetic.
Bond order = 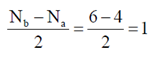
49. The pari which is isoelectronic is
(a) CN– and NO
(b) CN– and CO
(c) NO and CO
(d) CN– and O2–
Ans. (b)
Sol. The pair of species/ion/group which have same number of electrons are called isoelectronic pair
CN– = 6 + 7 + 1 = 14
CO = 6 + 8 = 14
O–2 = 16 + 1 = 17
NO = 7 + 8 = 15
Therefore, CN– and CO is an isoelectronic pair.
50. Complexes of Gd3+ are used in magnetic resonance imaging. The number of unpaired electrons in this ion is
(a) 1
(b) 3
(c) 5
(d) 7
Ans. (d)
Sol. The electronic configuration of Gd is [Xe] 4f7 5d1 6s2.
The electronic configuration of Gd3+ is [Xe] 4f7.
Hence, the number of unpaired electrons are 7 and magnetic moment is 7.94 BM.
51. Porphyrin in iron-porphyrin is
(a) bidentate
(b) tentradentate
(c) pentadentate
(d) hexadentate
Ans. (b)
Sol. The electronic configuration of Gd is [Xe] 4f7 5d1 6s2.
The electronic configuration of Gd3+ is [Xe] 4f7.
Hence, the number of unpaired electrons are 7 and magnetic moment is 7.94 BM.
52. For the gaseous reaction PCl5(g) 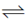 PCl3(g) + Cl2(g), the expression for the equiibrium constant Kpin terms of the degree of dissociation n and pressure P is
PCl3(g) + Cl2(g), the expression for the equiibrium constant Kpin terms of the degree of dissociation n and pressure P is
(a) 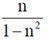
(b) 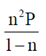
(c) 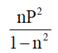
(d) 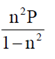
Ans. (d)
Sol. PCl5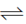 PCl3 + Cl2
PCl3 + Cl2
1 – n nn
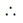 Total number of degree of dissociation
Total number of degree of dissociation
= 1 – n + n + n = 1 + n
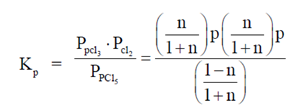
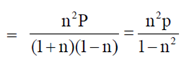
53. Heat capacity at constant volume CV for a monoatomic perfect gas is
(a) 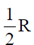
(b) R
(c) 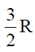
(d) 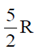
Ans. (c)
Sol. For monotaomic perfect gas only translation degree of freedom is present is :
Translation degree of freedom = 3
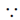 Heat capacity at constant volume, CV =
Heat capacity at constant volume, CV = 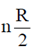

54. N2 + 3H2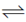 2NH3ΔH° = –3000J
2NH3ΔH° = –3000J
What happens when, under equilibrium conditions, temperature is decreased (Case I) or hydrogen is added (Case II)?
(a) Ammonia production increases in both the cases
(b) Ammonia production decreases in both the cases
(c) Ammonia production increases in Case I and ammonia dissociation increases in Case II
(d) Ammonia dissociation increases in Case I and ammonia production increases in Case II
Ans. (a)
Sol. 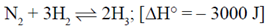
According to Le-Chatelier's principle, under equilibrium condition if temperature decrease in exothermic process, equilibrium shift to the forward direction and k increases. And addition to reactants (H2) in equilibrium mixture will favour forward direction. Hence, in both the cases ammonia production increases.
55. Ruby consists of low concentration of Cr3+ ions in place of Al3+ in alumina. Its red color is due to
(a) fluorescence
(b) phosphorescence
(c) radioactive decya
(d) chemiluminescence
Ans. (a)
Sol. A crystal of pure Al2O3 is known as corundum. In pure corundum, all electrons are paired and there is no absorption of light. Once one out of every hundred aluminium atoms is replaced by Cr-atom, negative charged oxygen ions surround the aluminium ion, so a chromium atom must donate three electrons to becomes Cr3+, replacing Al3+.
However, in Cr+3 there are partially filled energy levels or orbitals. It is these electrons that can be excited and that cause absorption of certain wavelength of light resulting in colour, i.e., its red colour arise due to fluorescence.
56. The acid dissociation constant of a weak acid HA is 1.0 × 10–5. The pH of 0.1 M solution of HA is
(a) 5.0
(b) 4.0
(c) 3.0
(d) 7.0
Ans. (c)
Sol. Given that
The acid dissociation constant of a weak acid
HA = 1.0 × 10–5
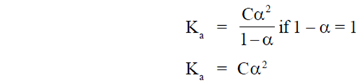


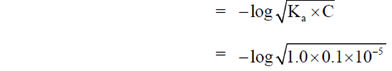
pH = 3
57. Standard redox potentials E° for three different redox reactions are given below :
Fe3+ + e 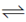 Fe2+ E° = +0.77V
Fe2+ E° = +0.77V
Cl2 + 2e 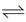 2Cl – E° = +1.36V
2Cl – E° = +1.36V
I2 + 2e 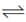 2I – E° = +0.53V
2I – E° = +0.53V
Under standard conditions, Fe3+ can oxidize
(a) I– to I2 but not Cl– to Cl2
(b) Cl– to Cl2 but not I– to I2
(c) Both I– to I2 and Cl– to Cl2
(d) Neither I– to I2 nor Cl– to Cl2
Ans. (a)
Sol. Fe3+ + e–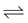 Fe2+;
Fe2+;
E° = + 0.77 V ..(i)
Cl2 + 2e–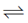 2Cl–; E° = + 1.36V ...(ii)
2Cl–; E° = + 1.36V ...(ii)
I2 + 2e–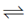 2l– ; E° = + 0.53 V ...(iii)
2l– ; E° = + 0.53 V ...(iii)
Greater the value of E°RP of an element, greater is the tendency of its reduction. In reaction (i) and (iii), (i) has greater E°RP so, Fe+3 can oxidise l– to l2 but not Cl– to Cl2.
58. Which one among the following is the correct structure for an optically active compound with a molecular formula C8H10O4 showing only three signals in its 1H NMR spectrum?
(a)
(b)
(c)
(d)
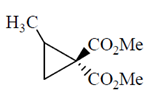
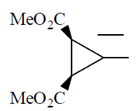
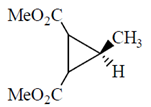
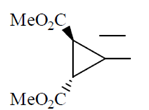
Ans. (c)
Sol.
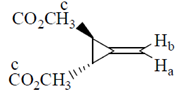
This is an optically active compound with molecular formula, C8H10O4 and also has diasterotropic carbon. And has three sets of equivalent proton which give three signal in 1H NMR spectroscopy.
59. Which one of the following will give more than one organisc compound upon ozonolysis followed by treatment with Ph3P?
(a) 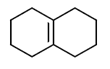
(b) 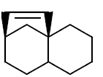
(c) 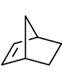
(d)
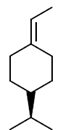
Ans. (d)
Sol.
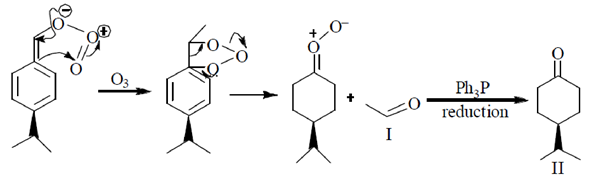
Therefore, (d) will give two organic compounds.
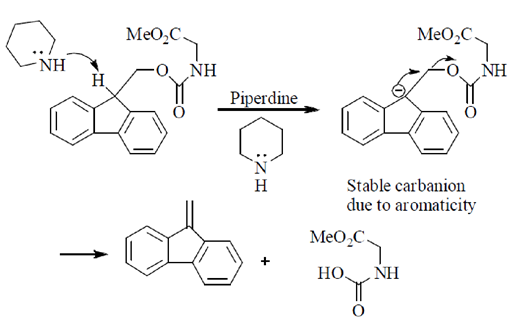
60. Identify the compound which will release glycine methy ester fastest on treatment with piperidine
(a) 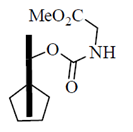
(b) 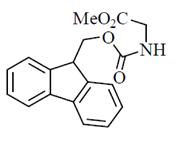
(c) 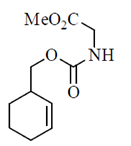
(d) 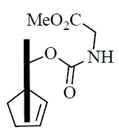
Ans. (b)
Sol.
61. Identify the major product formed in the following transformation :
(a) 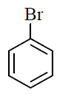
(b) 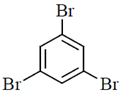
(c) 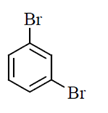
(d) 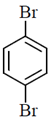
Ans. (b)
Sol.
62. How many CH3 groups are in equatorial position in the most stable conformation of the following molecule?
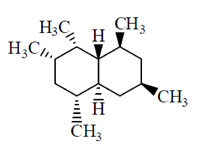
(a) 2
(b) 3
(c) 4
(d) 5
Ans. (c)
Sol.
63. Which one of the following compounds has peptide bonds?
(a) 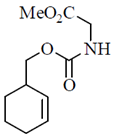
(b) 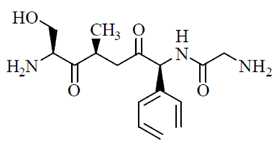
(c) 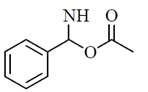
(d) 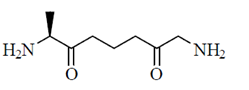
Ans. (b)
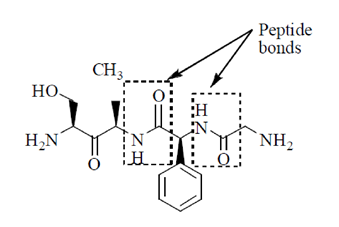
Sol. Peptides are compounds formed by the condensation of two or more same or different α-amino acids. The condensation occurs between amino acids with the elimination of water.
In this case, the carboxyl group of one amine acid and amino group of another amino acid gets condensed with the elimination of water molecule. The resulting 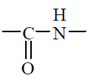 linkage is called peptide linkage or peptide bond.
linkage is called peptide linkage or peptide bond.
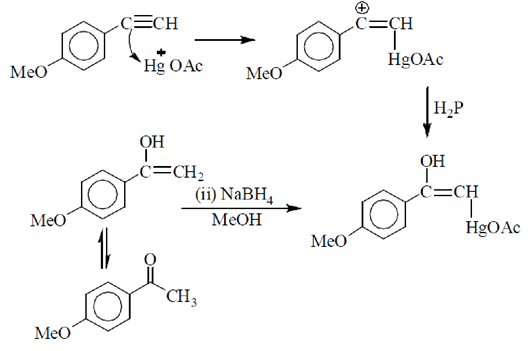
64. The condition under which the following transformation is carried out is
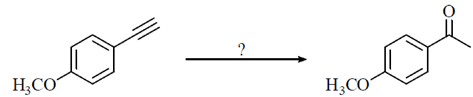
(a) (i) B2H6 (ii) NaOH/H2O2
(b) Hg(OAc)2, H2O
(c) (i) Hg (OAc)2, H2O (ii) NaBH4, MeOH
(d) H2, Pd-BaSO4
Ans. (c)
Sol.
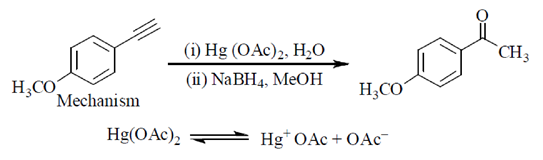
65. Dimension of viscosity is
(a) ML–2T–1
(b) ML–1T–1
(c) ML–1T–2
(d) ML–1T
Ans. (b)
Sol. Dimension of viscosity is given by 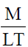 i.e. [ML–1T–1]
i.e. [ML–1T–1]
66. Consider a spherical organism of volume 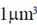 . Assume that the organism consists of only a fluid whose density is that of water. Calculate the gravitational force felt by this organisms on the surface of earth. Assume mass of earth = 6 × 1024 kg, radius of earth = 6 × 106m, density of water = 103 kg/m3 and gravitational constant G = 6 × 10–11 m3/(kgs2)
. Assume that the organism consists of only a fluid whose density is that of water. Calculate the gravitational force felt by this organisms on the surface of earth. Assume mass of earth = 6 × 1024 kg, radius of earth = 6 × 106m, density of water = 103 kg/m3 and gravitational constant G = 6 × 10–11 m3/(kgs2)
(a) 6 × 10–8 N
(b) 10–14 N
(c) 10–2 N
(d) 1N
Ans. (b)
Sol. Gravitational force (FG) is given by
FG = [symbols have their usual meaning]
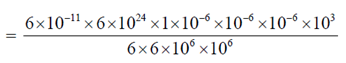
[mass of spherical organism = v × d = (10–6)3 × 103]
= 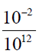 = 10–14N
= 10–14N
67. The graph below shows motion of a leopard over a period of 100s. The total distance covered by the leopard in this period is
(a) 4400 m
(b) 4000 m
(c) 2000 m
(d) 1000 m
Ans. (c)
Sol. Total distance can be calculated from velocity-time graph by finding the area under the velocity-time curve.
So, Distance = Area of triangle
= 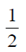 × 100 × 40 = 2000m
× 100 × 40 = 2000m
68. Consider a parallel plate capacitor with capacitance C0 = (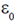 A/D) where
A/D) where 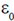 is the permittivity constant, A is the area of each plate and d is the separation between the plates. Suppose a dielectric substance of dielectric constant K is inserted fully between the plates of the capacitor. The new capacitance C after inserting the dielectric is given by
is the permittivity constant, A is the area of each plate and d is the separation between the plates. Suppose a dielectric substance of dielectric constant K is inserted fully between the plates of the capacitor. The new capacitance C after inserting the dielectric is given by
(a) 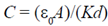
(b) 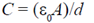
(c) C = C0/K2
(d) C = K1/2C0
Ans. (b)
Sol. Capacitance of parallel plate capacitor without the dielectric or air is given by
C = 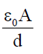
[A = Area of plate, d = Distance between plates]
When a dielectric of dielectric constant K is inserted, then capacitance of capacitor is given by
C = 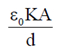
[rest see the derivation of capactiance of parallel plate capacitor with dielectric]
69. A plane electromagnetic wave is travelling along the negative x direction in free space. At a particular point in space and time, the value of 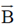 is 5 × 1019T
is 5 × 1019T 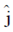 . The value of
. The value of 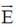 is equal to
is equal to
(a) 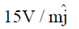
(b) 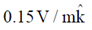
(c) 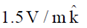
(d) 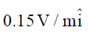
Ans. (c)
Sol. The relation between magnetic and electric field for an electromagnetic wave is given by
c = 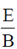
E = cB = 3 × 108 × 5 × 10–9 = 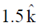
[direction is due to the fact that electric field E and magnetic field B of an electromagnetic wave is perpendicular 1 to each other]
70. Two coherent source S1 and S2 are vibrating in phase. The intensity of each of these sources is I0. For a path difference of n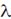 (n 0, 1, 2, ....) at any point P, the resultant intensity I is equal to
(n 0, 1, 2, ....) at any point P, the resultant intensity I is equal to
(a) I0
(b) 2I0
(c) 4I0
(d) 0
Ans. (c)
Sol. The intensity of the interference pattern for the given phase difference 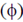 is given by [it does not depend upon path difference]
is given by [it does not depend upon path difference]
l = 
Since, phase difference is zero
So, l = 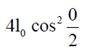
l = 4l0
71. In the circuit shown below, the current flowing through the ammeter A is equal to
(a) 2.0 A
(b) 0.2 A
(c) 1.5 A
(d) 36.2 A
Ans. (b)
Sol. The combination of resistance can be drawn as
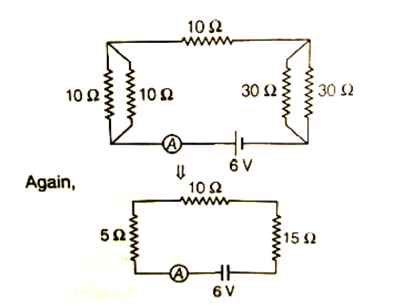
Now, all the resistors are in series
So, Req = 5 + 10 + 15 = 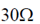
Current through the ammeter is given by Ohm's law,
i.e., l = 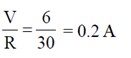
72. The figure below shows a rectangular loop of area A having just one turn and carrying a stady current I. The loop is placed in a uniform magnetic field B, and it rotates anti-clockwise. The torque acting on the loop is equal to
(a) 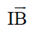
(b) 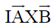
(c) 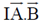
(d) 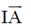
Ans. (b)
Sol. Torque produced in a coil placed in a magnetic field is given by
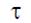 = NIA × B
= NIA × B
N = Number of turn in the coil
A = Area of the coil
B = Magnetic field
I = Current in the coil
73. In an experiment on photoelectric effect, the frequencies of incident radiations v1, v2, and v3 are such that v1 < v2 < v3. The stopping potentials for v1, v2, and v3 are V1, V2, and V3, respectively. Which one of the following is correct about the absolute values of V1, V2, and V3?
(a) V1 < V2 < V3
(b) V1 = V2 = V3
(c) V1 > V2 > V3
(d) v1V1 > v2V2 > v3V3
Ans. (a)
Sol. From Eintein's photoelectric equation
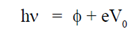
(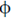 = constant for a particular metallic surface)
= constant for a particular metallic surface)
So, 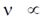 V0 (stopping potential)
V0 (stopping potential)
For 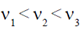 stopping potential should be
stopping potential should be
V1 < V2 < V3.
74. In 2/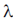 where
where 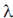 is the disintegration constant. The number of radioactive nuclei at time = in16/
is the disintegration constant. The number of radioactive nuclei at time = in16/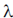 is
is
(a) 0N/4
(b) 0N/8
(c) 0N/16
(d) 0N/32
Ans. (c)
Sol. 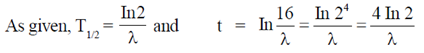
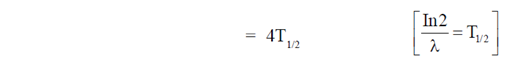
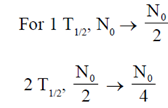
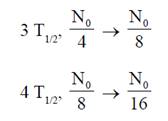
75. Consider two alternating circuits, circuit A and circuit B, as shown in the following figures.
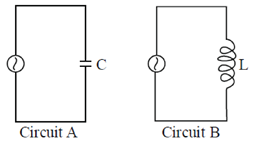
Choose the correct statement about these circuits
(a) Currents is 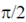 ahead of voltage in circuit B and current lags and the voltage by
ahead of voltage in circuit B and current lags and the voltage by 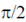 in circuit A
in circuit A
(b) Current is 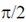 ahead of voltage in circuit A and current lags the voltage by
ahead of voltage in circuit A and current lags the voltage by 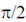 in circuit B
in circuit B
(c) Currents is 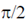 ahead of voltage in both the circuits
ahead of voltage in both the circuits
(d) Current lags the voltage by 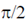 in both the circuits
in both the circuits
Ans. (b)
Sol. In case of a pure capacitive circuit, current leads voltage by 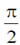 and in case of pure inductive circuit, voltage leads current by
and in case of pure inductive circuit, voltage leads current by 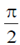 .
.
76. The current truth table for the following circuit is
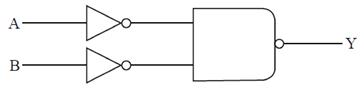
(a)
(b)
(c)
(d)
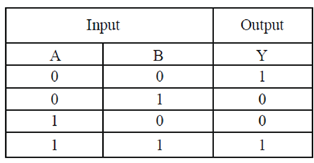
Ans. (c)
Sol.
77. The wireless communication frequency band for FM broadcase in India is
(a) 420–890 MHz
(b) 76–88 MHz
(c) 174–216 MHz
(d) 88–108 MHz
Ans. (d)
Sol. The wireless communication frequency band for FM broadcast in India is 88-108 MHz.
78. On a freezer, the maximum cooling temperature is marked as –40°F. What is the corresponding value on Centigrade scale?
(a) –328°C
(b) –24°C
(c) –9.8°C
(d) –40°C
Ans. (d)
Sol. As we know
Relation between °C and F is given by

79. A kinesin motor moves 8 nm for each ATP hydrolyzed. Hydrolysis of ATP results in releas of free energy which can be used for work. Suppose that hydrolysis of 1ATP molecule releases 20 KBt of free energy and kinesin motor is 100% efficient  , calculate the maximum force this motor can generate. Assume 1kBT = 4pN nm.
, calculate the maximum force this motor can generate. Assume 1kBT = 4pN nm.
(a) 20 pN
(b) 10 pN
(c) 5 pN
(d) 15 pN
Ans. (b)
Sol. Distance moved by kinesin motor
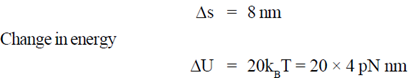
Change in energy
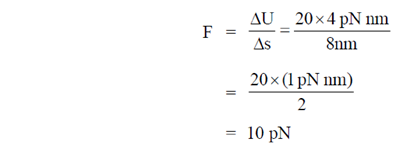
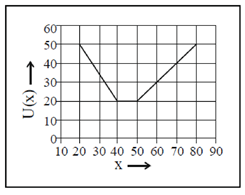
80. The potential energy function U(x) for a particle is shown below. The force corresponding to this potential is
(a)
(b)
(c)
(d)
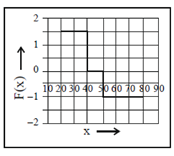
Ans. (d)
Sol. As we know that
Force, F = 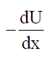
During 20 to 40 units of x
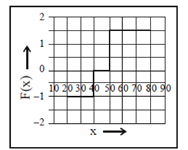
Force, F = 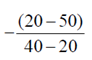
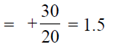
During 40 to 50 units of x
F = 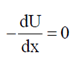
During 50 to 80 units of x
F = 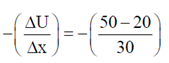
= –1N
These values correspond to graph in option (d)
81. A 2m long rigid rod is placed symmetrically on a pivot and two masses are hung on the left side of the pivot as shown in figure. Calculate the mass required to be hanged at a distance of 1m from pivot (on the right hand side of the rod) to keep the rod horizontal.
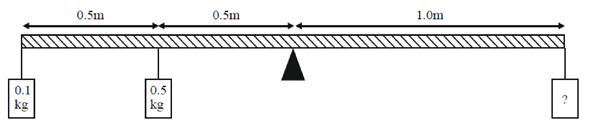
(a) 0.60 kg
(b) 0.35 kg
(c) 0.30 kg
(d) 0.10 kg
Ans. (b)
Sol. By balancing the torque produced by the masses,
i.e., 0.5 × 0.5 + 0.1 × 1 = m × 1
 = 0.25 + 0.1 = 0.35
= 0.25 + 0.1 = 0.35
82. A rectangular piece of aluminium has length 100cm, width 10cm and height 5cm. A force of 7 × 105N stretches it along its length. The strain of the rod is (assume Young's modulus of aluminium to be 7 × 1010N/m2)
(a) 2 × 103
(b) 2 × 10–3
(c) 2 × 10–1
(d) 2
Ans. (b)
Sol. As we know that
Young's modulus = 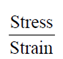
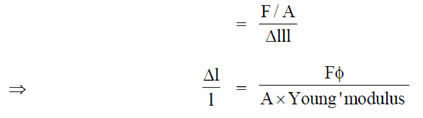
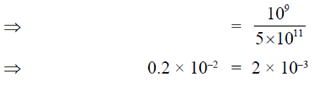
83. Evaluate the intergral 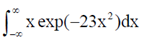
(a) 1
(b) 0
(c) 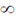
(d) 1/23
Ans. (b)
Sol. Let, l = 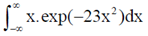
Let f(x) = 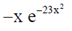
Then, f(–x) = 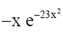 = –f(x)
= –f(x)
 f(x) is an odd function
f(x) is an odd function
Hence, l = 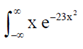 dx = 0
dx = 0
[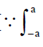 f(x) dx = 0, if f(x) is an odd function]
f(x) dx = 0, if f(x) is an odd function]
84. Let d1 and d2 be determinants of two matrices as given below :
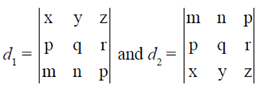
Assuming d1 and d2 to be non-zero, the correct relationship between them is
(a) d1 = d2
(b) d1 = 2d2
(c) d1 = 2d2
(d) d1 = –d2
Ans. (d)
Sol.
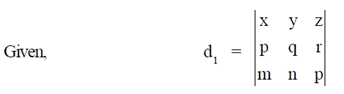
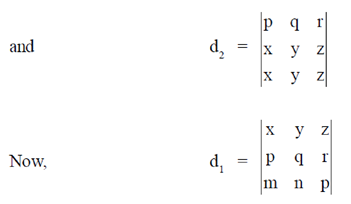
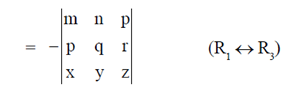
= –d2
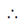 d1 = –d2
d1 = –d2
85. In (exp (Inxa)) =
(a) a ln (x)
(b) a exp (x)
(c) ax
(d) ln (a ln x)
Ans. (a)
Sol. In {exp (In xa)}
= 
= log(xa)
= log xa
= a log x 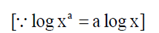
86. Many biological molecules are helices. Assuming that t is a continuous parameter between 0 and 1000, which set of the following equations represent a helix?
(a) x = 3t, y = 3, z = 5t
(b) x = 3cos t, y = 3sin t, z = t
(c) x = 3sin t, y = 5, z = 5sin t
(d) x = 3cos t, y = 3sin t, z = 5t
Ans. (d)
Sol. We know that, the parametric equation for a 3D helix is
x = R cos t
y = R sin t
z = ht
where, h is the height.
87. Evaluate 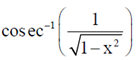
(a) cos–1(x)
(b) cosec–1(x)
(c) sin–1(x)
(d) tan(x)
Ans. (a)
Sol.
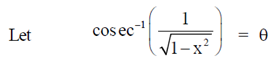
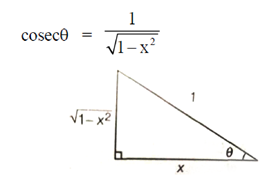
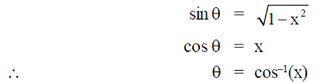
88. Consider a population of N haploid individuals, 10% of the gene A, while the rest do not have this gene. The total number of offspring in the next generation is also N. If the distribution of the chromosomes in the next generation is binomial, the probability that no offspring will have gene A is
(a) 1C1 (0.1)N
(b) 0.9N
(c) 9C1 (0.9)N
(d) 0.1N
Ans. (b)
Sol. Let  denotes the offspring with gene A.
denotes the offspring with gene A.
p = 10% = 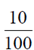 = 0.1
= 0.1
and q = 1 – p = 1 – 0.1 = 0.9
 P(
P( = 0) = NC0 p0qN
= 0) = NC0 p0qN
= NC0(0.1)0(0.9)N
= 1 × 1 × (0.9)N = (0.9)N
89. The value of 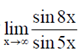 is
is
(a) 8/5
(b) 0
(c) 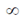
(d) 5/8
Ans. (a)
Sol.
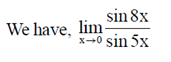
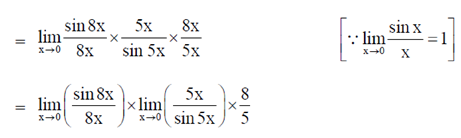
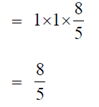
90. You are picking a random number r from a uniform distribution between 0 and 1. The probability that r is between 0.5 and 0.7 is
(a) 0.2
(b) 1/2
(c) 1/7(d) 0.1
Ans. (d)
Sol. Required probability = 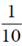 = 0.1
= 0.1
91. A bacterium of mas m moving in a fluid feels a viscous drag ∝. The velocity of the bacterium obeys the following differential equation m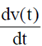 = –∝ v(t). Given that v(0) is the velocity at time t = 0, v(t) is equal to
= –∝ v(t). Given that v(0) is the velocity at time t = 0, v(t) is equal to
(a) 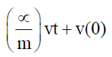
(b) v(0) ln v(t)
(c) 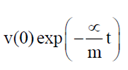
(d) 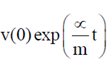
Ans. (c)
Sol. Given differential equation is
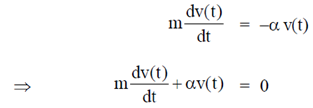
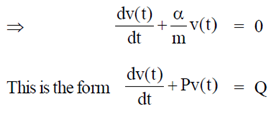
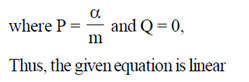
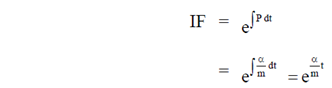
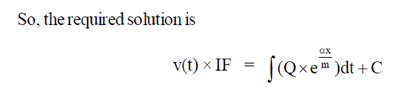
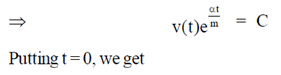

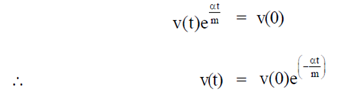
92. Match the types of matrices listed in Column I with the representative examples listed in Column II
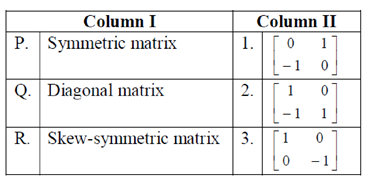
(a) P–3, Q–3, R–1
(b) P–1, Q–3, R–3
(c) P–3, Q–1, R–1
(d) P–2, Q–3, R–3
Ans. (c)
Sol. 
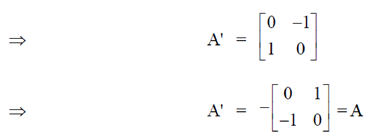
 A is skew-symmetric
A is skew-symmetric
Also, it is diagonal matrix
Let B = 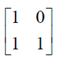
It is a diagonal matrix
B' = 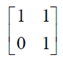
It is neither symmetric nor skew-symmetric matrix
Let C = 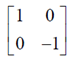
C' = 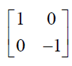 = C
= C
 C is symmetric mayrix.
C is symmetric mayrix.
93. Consider the function f(x) = Ax4 – Bx2 such that A > 0 and B > 0. Which of the following statements are true?
P : The function has a maximum at x = 0
Q : The function has a minimum at x = 0
R : The value of the function is zero at x = 0
S : The value of the function non-zero at x = 0
(a) Only Q and S
(b) Only P and R
(c) Only P and S
(d) Only Q and S
Ans. (b)
Sol. Given, f(x) = Ax4 – Bx2
 f'(x) = 4Ax3 – 2Bx
f'(x) = 4Ax3 – 2Bx
For maxima or minima,
 f' (x) = 0
f' (x) = 0
 4Ax3 – 2Bx = 0
4Ax3 – 2Bx = 0
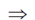 x(4Ax2 – 2B) = 0
x(4Ax2 – 2B) = 0
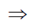 x = 0 or x2 =
x = 0 or x2 = 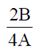
x = 0 or x = 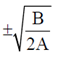
Now, f"(x) = 12Ax2 – 2B
f"(0) = –2B < 0
f(x) has maxima at x
Clearly, f(0) = 0
94. Evaluate the derivative 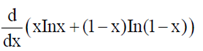
(a) 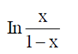
(b) 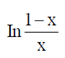
(c) 0
(d) 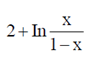
Ans. (d)
Sol. 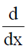 [x In x + (1 – x) In (1 – x)]
[x In x + (1 – x) In (1 – x)]
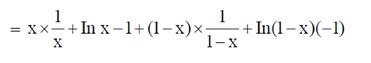
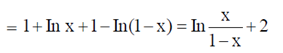
95. The operation 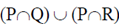 for sets P, Q and R can also be written as
for sets P, Q and R can also be written as
(a) 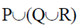
(b) 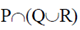
(c) 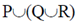
(d) 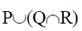
Ans. (b)
Sol. 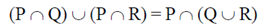
[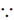 union and intersection are distributive]
union and intersection are distributive]
96. The number of roots of the polynomial equation 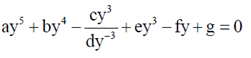
(a) 4
(b) 3
(c) 6
(d) 5
Ans. (c)
Sol. Given, ay5 + bu4 – 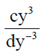 + ey2 – fy + g = 0
+ ey2 – fy + g = 0
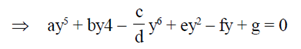
Since, the degree of polynomial is 6.
 Number of roots = 6
Number of roots = 6
97. The complex number
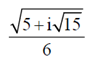
when converted into polar form would be
(a) 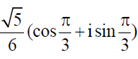
(b) 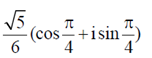
(c) 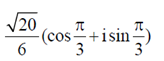
(d) 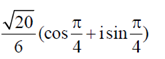
Ans. (c)
Sol. Given,
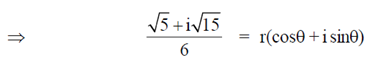
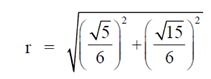
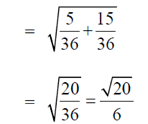

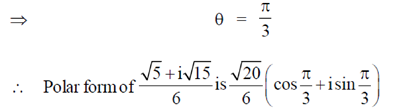
98. A population of rats increases every year. Each year, increase in the population is twice that of the previous year. If the population is 1000 in 2013 and it increases to 3000 in 2014, what will be the number of rats in 2018?
(a) 12000
(b) 31000
(c) 63000
(d) 32000
Ans. (c)
Sol.
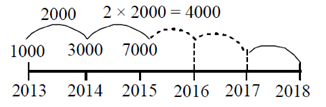
Let tn denotes the population of rats in nth year. Also, assume 1st year as 2013. Then, we need to find t6, i.e. population of rats is 2018.
Consider, Sn = 1000 + 3000 + 7000 + ... + tn ...(i)
Then, this can be rewritten as
Sn = 1000 + 3000 + ... + tn–1 + tn ...(ii)
On subtracting Eq. (ii) from Eq. (i), we get
 0 = 1000 + 2000 + 4000 ... + (tn – tn–1) – tn
0 = 1000 + 2000 + 4000 ... + (tn – tn–1) – tn
 tn = 1000 + 2000 + 4000 + ... + (tn – tn–1) ...(iii)
tn = 1000 + 2000 + 4000 + ... + (tn – tn–1) ...(iii)
tn = 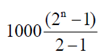
[ series (iii) from a GP with first ferm a = 1000 and r = 2]
series (iii) from a GP with first ferm a = 1000 and r = 2]
 tn = 1000 (2n – 1)
tn = 1000 (2n – 1)
For n = 6, we have
t6 = 1000 (26 – 1)
 t6 = 1000 (64 – 1)
t6 = 1000 (64 – 1)
 t6 = 63000
t6 = 63000
Hence, there are 63000 rats in 2018.
99. The correct equation for the ellipse shown below is
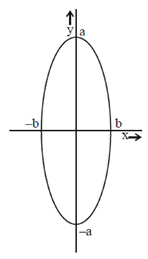
(a) 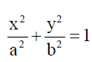
(b) 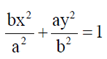
(c) 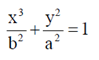
(d) 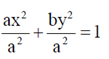
Ans. (c)
Sol. Since, the given ellipse is vertical with length of major axis = 2a and length of minor axis = 2b
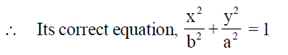
100. The life-spans of three cells in a particular experiment are 3, 6 and 9 days. The standard deviation in the life-span is
(a) 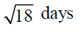
(b) 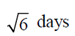
(c) 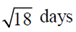
(d) 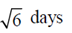
Ans. (b)
Sol. Let 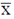 be the mean of given life-spans, then
be the mean of given life-spans, then
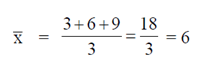
Now, strandard deviation in the life-span is given by
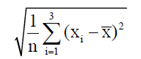
where n = 3, x1 = 3, x2 = 6 and x3 = 9,
Hence, the strandard deviation in the life-span