IIT JAM Biology 2012
Previous Year Question Paper with Solution.
1. The % bae pair values of four nucleic samples are provided below. Which one of the following samples has the highest Tm?
(a) A = 31; T = 21; G = 20; C = 28
(b) A = 26; T =14; G = 34 ; C = 26
(c) A = 17; T = 19; G = 33; C = 31
(d) A = 20; T = 30; G = 25; C = 25
Ans. (c)
Sol. Melting temperature (Tm) is the temperature at which half of the DNA strands are in the random coil or single-stranded (ssDNA) state.
The nucleotide pair 'A-T' has a weaker bond than the nucleotide pair 'G-C'.
To predict the highest Tm for the given % base pair we will follow 2 + 4 rule of thumb. i.e. assign 2°C to each A-T pair and 4°C to each G-C pair. Therefore,
(i) For A = 31, T = 21, G = 20, C = 28 we have
Tm = 2(31 + 21) + 4(20 + 28)
= 104 + 192 = 296°C
(ii) For A = 26, T = 14, G = 34, C = 26, we have
Tm = 2(26 + 14) + 4(34 + 26)
= 80 + 240 = 320°C
(iii) For A = 17, T = 19, G = 33, C = 31, we have
Tm = 2(17 + 19) + 4(33 + 31)
= 72 + 256 = 328°C
(iv) For A = 20, T = 30, G = 25, C = 25, we have
Tm = 2(20 + 30) + 4(25 + 25)
= 100 + 200 = 300°C
Thus, option (c) has the highest Tm.
2. Which one of the following is TRUE regarding organization of human chromosomes? It is made up of
(a) histones that are acidic proteins
(b) extra-chromosomal circular DNA
(c) chromatin that consists of DNA and basic proteins
(d) non-chromosomal DNA
Ans. (c)
Sol. Human chromosomes are made up of chromation, i.e., a complex of macromolecules found in cells, consisting of DNA and basic proteins.
3. The melting point of unsaturated fatty acid
(a) is not related to the number of double bonds
(b) increases with increase in the number of double bonds
(c) is higher than that of its corresponding saturated fatty acid
(d) decreases with increase in the number of double bonds
Ans. (d)
Sol. An unsaturated fatty acid is a one in which there occurs atleast one double bond within the fatty acid chain. They may be mono, tri, tetra or polyunsaturated fatty acids. Polyunsaturated are often known as unsaturated fats, due to many double bonds. They provide more fluidity and are usually liquid at room temperature. Thus, the polyunsaturated or unsatruated fats have the lowest melting point when compared to monounsaturated and saturated fatty acids. Hence, the melting point of unsaturated fatty acids. Hence, the melting point of unsaturated fatty acids acids decrease with the increase in the number of double or degree of unsaturation.
4. Match the hormones in Group I with the metabolic processes in Group II
Group I Group II
P. Progesterone 1. Increase gluconeogenesis in liver
Q. Glucagon 2. Implantation of fertilized ovum
R. Insulin 3. Stimultes spermatogenesis process
S. Androgen 4. Stimulates glucose uptake and storage
(a) P–2, Q–1, R–4, S–3
(b) P–3, Q–2, R–1, S–4
(c) P–1, Q–4, R–2, S–3
(d) P–1, Q–2, R–4, S–3
Ans. (a)
Sol. Progesterone is an endogeneous steroid female hormone involved in the menstrual cycle, (i.e., important for ovulation and menstruation). It also plays a role in maintaining pregnancy. It is produced in ovaries in females. It helps to prepare females body for pregnancy and implantation of fertilised ovum.
Glucagon is the hormone secreted by the lapha cells of pancreas. It works opposite to insulin by increasing gluconegenesis in liver. Insuline, on the other hand, is the hormone produced by the beta cells of pancreas. It plays a key role in the regulation of blood glucose levels. It stimulates uptake of glucose and its storage in the body.
Androgen, is the steroid hormone that stimulates or controls the development and maintenance of male characteristics and stimulates spermatogenesis in vertebrates by binding to androgen receptors.
5. The most abundant immunoglobulin in human blood is
(a) IgM
(b) IgA
(c) IgD
(d) IgG
Ans. (d)
Sol. Comprising upto 80% of the total antibodies found in human body, IgG is the smallest, yet most abundant antibody found in human body and that of other mammals too. It is found in all bodily fluids, secretions, etc. It is the only antibody that protects a foetus by passing through the mother's placenta.
6. The process of purification and recovery of a product in biotechnology is known as
(a) upstream processing
(b) downstream processing
(c) incubation
(d) formulation
Ans. (b)
Sol. Downstream processing is known as the process that has the primary aim of purification and recovery of target product of required specification in biotechnology. It involves all the processes following fermentation process. The target product in this, may be recovered by processing the cell if it is intracellular or by processing the spent medium if it is extracellular.
7. If the velocity of an enzyme catalyzed reaction is 60% of vmax, then the ratio of substrate concentration [S] to Michaelis-Menton constant KM is
(a) 1
(b) 1.5
(c) 2
(d) 4
Ans. (b)
Sol. According to the Michaelis-Menten equation


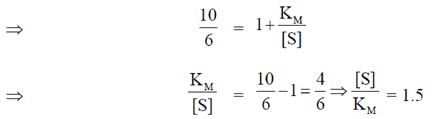
8. In a DNA replication experiment, 1μg of 15N DNA is allowed to replicate till two energies with 14N DNA. The amount (in μg) of 14N DNA formed during the second replication process is
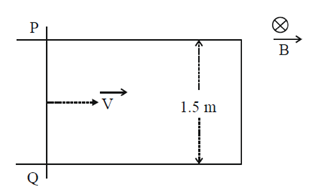
(a) 1
(b) 2
(c) 3
(d) 4
Ans. (b)
Sol. DNA replication is the process of producing two identical molecules from one original DNA molecule. This biological process occurs in all living organisms. It is the basis for biological inheritance. It is also known as semiconserdvative replication because the DNA molecule in this process, serves as a template for the production of the complementary strand. If 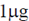 of 15N is replicated till two generations with 14N DNA, in second generation, the amount of 14N DNA formed will be 2mg.
of 15N is replicated till two generations with 14N DNA, in second generation, the amount of 14N DNA formed will be 2mg.
9. Transport activities in cell membranes are carried by ..................; whereas fluidity of membranes is maintained by ..................... .
(a) lipids; proteins
(b) proteins; nucleic acids
(c) lipids; nucleic acids
(d) proteins, lipids
Ans. (d)
Sol. The cell membrane, also known as plasma membrane or cytoplasmic membrane, is a biological membrane that separates the interior of all cells from the outside environment. The basic function of the cell membrane is to protect the cell from its surroundings. Cell membrane has a large content of proteins, typically around 50% of membrane volume. They are important for cell because they are responsible for various biological activities, while, lipids in the cell membrane are responsible for regulating the fluidity by altering lipid composition.
10. Nodules of legminous plants are a good source for the isolation of bacteria capable of
(a) nitrogen fixation
(b) carbon fixation
(c) cellulalse production
(d) amylase production
Ans. (a)
Sol. Leguminous plants are plants with root nodules, which are the extra lobes in the roots of certain plants, such as peas, and bacteria, in which the bacteria stay. These plants need root nodules because the bacteria in these nodules convertes nitrogen into nitrates and give it to the plant. The plant, thus, uses this nitrogen to make protein and other stuff. In return, the plants provide shelter and carbohydrates to the bacteria.
11. Which of the following statements regarding techniques and their applications is NOT correct?
(a) Recombinant DNA Technology : cloning genes and expression for proteins
(b) Enzyme Linked Immuno absorbent Assay : recognize antigen and antibody interactions
(c) Polymerase Chain Reaction : amplify specific DNA sequences
(d) Western Blot : def8ect DNA is given samples
Ans. (a)
Sol. Recombinant DNA technology is the phenomenon for technique of joining together of DNA molecule from two different species that are inserted into a host organism to produce new genetic combinations or a hybrid that are of produce new genetic combinations or a hybrid that are of value to science, agriculture, medicine, genetics etc. It is useful to diagnose various diseases, to produce monoclonal antibodies, DNA fingerprinting, gene therapy, production of vaccines, etc.
12. Addition of casein to solid media and picking up bacterial colonies that form clean zone is termed as
(a) different enrichment
(b) streaking
(c) serial dilution
(d) selective enrichment
Ans. (c)
Sol. Streaking or streak plate formation involves the progressive dilution of an inoculum of bacteria or yeast over the surface of solidified medium then the result is that some of the colories on the plate grow well separated from each other and form clear zone. These now can be separated easily.
13. Leishmaniasis is transmitted by
(a) sand fly
(b) tsetse fly
(c) rodent fly
(d) mosquitoes
Ans. (a)
Sol. Leishmaniasis is a disease caused by protozoan parasites of the genus Leishmania and spread by the bite of certain types of the disease, i.e. cutaneous, visceral and mucocutaneous.
Infection in humans are caused by mote than 20 species of Leishmania. It can be partly prevented by sleeping under nets treated with insecticide, spraying insecticides etc.
14. The binding of oxygen to hemoglobin is affected by
(a) hemoglobin concentration
(b) partial pressure of oxygen
(c) bicarbonate concentration
(d) 2, 3-biphosphoglyceric acid
Ans. (b)
Sol. Haemoglobin is a red coloured pigment found in red blood cells that can bind with oxygen. It is a protein made up of four polypeptide subunits, within each subunit is a heme group, which is an iron contaiing ring structure that has the ability to bind to oxygen. However, this ability of haemoglobin to pick up or release oxygen also depends on the pO2 (i.e., the partial presence of O2 in this environment). When the partial pressure of O2 (pO2) is high, as it is in the capillariesaround the lung, each molecule of haemoglobin can carry its maximum lead of four oxygen molecules. As the blood circulates around the body, the blood experiences the blood circulates around the body, the blood experiences lowe levels of partial pressure. At these low levels of pO2, the haemoglobin releases some of the O2 it is carrying.
15. The Human Genome Project was aimed for
(a) DNA sequencing and DNA mapping
(b) Protein and DNA sequencing
(c) Protein sequencing and DNA mapping
(d) RNA sequencing and genome database
Ans. (a)
Sol. Human Genome project was the international, collaborative research program whose goal was the complete mapping and understanding of all the genes of human beings. All our genes are known as 'genome'. The main aim of the human genome project was to provide a complete and accurate sequence of the three billion DNA base pairs that make up the human genome. It originally aimed to map the nucleotides contained in a human haploid reference genome (more than three billion). The genome of any given individual is unique. Mapping the human genome involves sequencing multiple variations of each gene.
16. In photosynthesis, the light energy is used to
(a) generate low energy electrons
(b) produce ATP and NADPH
(c) generate chlorophyll
(d) form water from oxygen
Ans. (b)
Sol. Photosynthesis is a process of converting light energy from sun into chemical energy that can be later released to fuel the organisms and storing it in the bonds of sugar. This process occurs in plants and some algae and cyanobacteria. Such organisms are called photoautrophs. It helps to maintain the atmospheric oxygen levels and supplies all the organic compounds of most of the energy necessary for life on Earth. In these light dependent reactions, some energy is used to strip electrons from suitable substances, such as water, producing oxygen gas. Further two more compounds are generated, i.e. NADPH and ATP (energy currency of cell).
17. In gram staining of gram negative bacteria, the crystal violet-iodien complex formed will be washed away after addition of
(a) safranin solution
(b) ethyl acetate
(c) water
(d) alcohol
Ans. (d)
Sol. Photosynthesis is a process of converting light energy from sun into chemical energy that can be later released to fuel the organisms and storing it in the bonds of sugar. This process occurs in plants and some algae and cyanobacteria. Such organisms are called photoautrophs. It helps to maintain the atmospheric oxygen levels and supplies all the organic compounds of most of the energy necessary for life on Earth. In these light dependent reactions, some energy is used to strip electrons from suitable substances, such as water, producing oxygen gas. Further two more compounds are generated, i.e. NADPH and ATP (energy currency of cell).
18. The oxidation of glycolate to glyoxylate during photorespiration occurs in
(a) bundle sheath cells
(b) mesophyll cells
(c) mesenchymal cells
(d) parenchmal cells
Ans. (a)
Sol. Photorespiration is a process in plant metabolism which attempts to reduce the consequences of wasteful oxygenation reaction by the enzyme RuBisCo. This process reduces the efficiency of photosynthesis, potentially reducing photosynthetic output by 25% in C3-plants. The oxidation of glycolate to glycolate during photorespiration occurs in peroxisomes of bundle sheath cells.
19. In higher plants, the light harvesting molecules are
(a) vitamin D and cytochrome C
(b) cytochrome C and chlorophyll
(c) anthocyanin and carotenoid
(d) chlorophyll and carotenoid
Ans. (d)
Sol. A during harvesting complex is a complex of subunit proteins that may be the part of a larger supercoplex of a photosystem, the functional unit in photosynthesis. It is used by plants and photosynthesis. It is used by plants and photosynthetic bacteria to collect more of the incoming reaction centre alone. These complexes are found in a wide variety amont the different photosynthetic species. They consists of proteins and photosynthetic pigments and surround a photosynthetic reaction center to focus energy, obtained from photons absorbed by the pigment, toward the reaction center. Light harvesting complexes are located around the reaction center and additional pigments, chlorophyll, and carotenoids to funnel absorbed energy to the special pair via resonance energy transfer.
20. Match the cell organells in Group I wth their functions listed in Group II
Group I Group II
P. Peroxisome 1. storage of starch granules
Q. Mitochondria 2. detoxification
R. Ribosome 3. proton gradient formation
S. Leucoplast 4. protein synthesis
(a) P–3, Q–2, R–1, S–4
(b) P–2, Q–4, R–3, S–1
(c) P–2, Q–3, R–4, S–1
(d) P–1, Q–3, R–4, S–2
Ans. (c)
Sol. Peroxisomes are the small, membrane enclosed cell organelles found in plants and animal cells. It contains enzymes involved in a variety of metabolic reactios, including several aspects of energy metabolism. In liver and kidney cells, peroxisomes detoxify various toxic substances that enter the blood.
Mitochondria, is an organelle found in large number in most cells, in which the biochemical processes of respiriation and energy production occur. It is a double membranous structure whose inner part is at folded inwards to form cristae. The elctrochemical proton gradient across the inner mitochondrial membrane is used to drive ATP synthesis in the critical process of oxidative phosphorylation.
Ribosomes are the protein builders or the protein synthesisers of the cell. They connect one amino acid at a time and build long chains. These are found in both prokaryotes and eukaryotes. These are known as the factory for 'protein synthesis' (i.e., translation).
Leucoplasts are the type of plastids found in the cytoplasm of only plant cells. It is a colourless plastid responsible for the manufacturing and storage of a starch granules.
21. The effect of hypotonic solution on a plant cell and red blood cell are, respectively,
(a) turgid and burst
(b) shrink and burst
(c) turgid and shrink
(d) plasmolysed and burst
Ans. (a)
Sol. A hyptonic solution is any solution that has a lower osmotic pressure than another solution. It is a solution having less solute and more water than other solution. If any plant cell or a RBC is placed in a hypotonic solution (i.e. pure water) they would swell up and burst because of the movement of water across the cell membrane. In this condition, the water would move into the cell to try to dilute the solution inside the cell. This would continue until the cell bursts.
22. Which one of the following statements is NOT correct for the classification of carbohydrates?
(a) Dihydroxyacetone and glyceraldehyde are trioses
(b) Galactose and glucose are hexoses
(c) Mannose and fructose and pentoses
(d) Erythrose and threose are tetroses
Ans. (c)
Sol. Carbohydrates are one of the main types of nutrients. They act as a major source of energy for our body. Our digestive system changes carbohydrates into glucose (blood sugar) which is further used for energy for cells, tissues and organs. These are classified into three main types, i.e., monosaccharides, oligosaccharides and polysaccharides.
Monosaccharides are often called simple sugars, consisting of single aldehyde or ketone units. Their general formula is Cn(H2O)n. They may further be of following types :
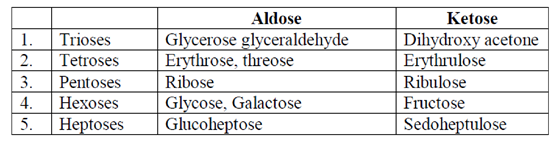
Hence, all the options are correct excepting mannose and fructose because, the former is a C-2 epimer of glucose while the latter is a hexose.
23. The last stage of spermatozoa formation in spermatogenesis is
(a) second meiotic division
(b) first meiotic division
(c) mitosis
(d) differentiation
Ans. (a)
Sol. Spermatogenesis is the process of development of a sperm cell or spermatozoa in the testis, which originates from the Primordial Germ cells (PGCs).
The complete process of spermatogenesis comprises of four different phases, i.e. multiplication phase, growth phase, maturation phase and differentiation phase.
Maturation phase or phase of maturation of spermatids is the phase in which some of the spermatogonia called primary spermatocytes undergo meiosis. These primary spermatocytes undergo first meiotic division and produce secondary spermatocytes (23 chromosomes) which further undergoes secondary meiotic division ton produce spermatids (i.e. the last stage of spermatogenesis).
24. In plant tissue culture, differentiation of callus to root requires
(a) high auxin and low cytokinin
(b) low auxin and high cytokinin
(c) low auxin and low cytokinin
(d) high auxin and high cytokinin
Ans. (d)
Sol. Plant development is a series of biochemical events which ultimately lead to morphogenic changes. The process is regulated by a number of phytochromes which bring about differential gene activity leading to the development of specific phenotype.
The interaction between various homones is very complex and they operate at different levels like differential transcription, protein synthesis, enzyme activity, permeability etc. Callus is any mass of an unorganised parenchyma cells derived from plant tissue (explants) for the use in biological research and biotechnology. It is like a cancerous tumour, where the cells are undifferentiated and they are under the spell of uncontrolled mitotic activity. If the ratio between auxin and cytokinin is high, the callus undergoes tranformation and produce roots, while if this ratio becomes low, the callus cells initates shoots formation.
25. Regenerative medicine aims at
(a) discovering small molecules
(b) generating therapeutic proteins
(c) growing tissues and organs
(d) identify genetic mutations
Ans. (c)
Sol. Regenerative medicine is a branch of translational reserach in tissue engineering and molecular biology which deals with the process of replacing, engineering or regenerating human cells, tissues or organs to restore or establish normal function. It also includes the possibility of growing tissues and organs in the laboratory and safetyv implanting them when the body cannot heal itself.
26. Which of the following is NOT required in a Polymerase Chain Reaction?
(a) DNA template
(b) MG++ ion
(c) Primers
(d) Restriction enzymes
Ans. (d)
Sol. PCR or polymerase Chain Reaction is a technology in molecular biology which is used to amplify a single or few copies of a piece of DNA across several orders of magnitude generating thousands to millions of copies of a particular DNA sequence. For PCR reaction of occur, number of things that are required are DNA template, primers, Taq polymerase, Mg2+, etc. Hence, restriction enzyme does not play any role in this reaction because they are the moelcular scissors that cut a DNA molecule at a particular place, i.e. near specific recognition nucleotide sequences known as restriction sites.
27. Which one of the following processes allows introduction of gene of interest to a target site in genome?
(a) Somatic embroygenesis
(b) Organogenesis
(c) Gene cloning
(d) Southern
Ans. (c)
Sol. Gene cloning is the process in which a gene of interest is located and copied (cloned) out of DNA extracted from an organism. When DNA is extracted from an organism, all of its genes are extracted at one time. This DNA contains thousands of different genes.
28. Based on the dissociation constant Kd, the protein-ligand pair that has the strongest interactions is
(a) insulin and insulin receptor (Kd = 1 × 10–10)
(b) avidin and biotin (Kd = 1 × 10–15)
(c) HIV surface protein and anti-HIV IgG (Kd = 4 × 10–10)
(d) Calmodulin and calcium (Kd = 3 × 10–6)
Ans. (b)
Sol. A dissociation constant (Kd) is a specific type of equilibrium constant tat measures the propensity of a larger object to separate reversibly into smaller components. Biotin and avidin bind with a dissociatino constant of 10–15 M = 1 fM = 0.000001 nM which makes them a protein-ligand pair with the strongest interaction.
29. In genetic code, the codon degeneracy occurs at ................... position (s)
(a) first
(b) second
(c) third
(d) first and third
Ans. (c)
Sol. The genetic code is the set of rules by which information encoded within genetic material (DNA or mRNA) sequences is translated into proteins by living cells. Degeneracy, is the redundancy of the genetic code. It is often said to be degenerated if any nucleotide at its third position specifies the same amino acid.
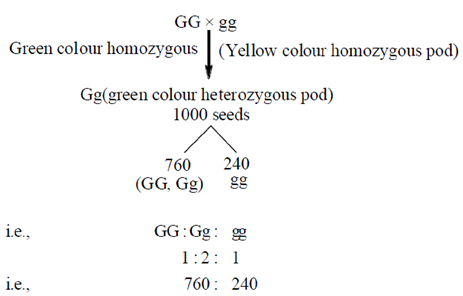
30. In pea plants, green pod colour is dominant over yellow pod color. 1000 seeds taken from a peaplant germinated to produce 760 green pod plants and 240 yellow pod plants. the parental genotype and phenotype of the seed plants are
(a) heterozygous and yellow
(b) homozygous and green
(c) hetermozygous and green
(d) homozygous and yellow
Ans. (c)
Sol. The given situation is expressing the law of dominance given by Mendel. According to this law, the recessive alleles will always be masked by the dominant alleles. Therefore, a cross between homozygous dominant and homozygous recessive will always express the dominant phenotype while, still having a heterozygous genotype. Thus, if in the given situation, green colour pod (GG) is dominant over yellow colour pod (gg), and 1000 seeds are taken from plant germinated to produce 760 and 240 pods of green and yellow respectively, the parental genotype and phenotype of the parent plants will be, heterozgous and green (i.e. Gg)
31. Which of the following is FALSE for DNA?
(a) DNA strands do not contain Uracil
(b) Two stands of DNA associate in parallel arrangement
(c) Orientation of one strand is 3' to 5' and other strand is 5' to 3'.
(d) Ability of nucleotide in two strands to form specific base pairs is due to hydrogen bonds
Ans. (b)
Sol. DNA or deoxyribonucleic acid is the hereditary material in humans and almost all other organisms. Most DNA is located in the cell nucleus, but a small amount of DNA can also be found in the mitochondria. The two strands of DNA associates in an antiparallel arrangement, i.e. 3' 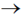 5' and 5'
5' and 5' 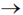 3' orentation. Nucleotides in both DNA strands forms specific base pairs due to hydrogen bonding.
3' orentation. Nucleotides in both DNA strands forms specific base pairs due to hydrogen bonding.
32. In 2009, the swine flu outbreak was ................... in nature.
(a) sporadic
(b) pandemic
(c) chronic
(d) endemic
Ans. (b)
Sol. The swine flu or the 2009 pandemic was an influenza pandemic and was known to be the second of the two pandemics involving H1N1 influenza virus. It was first described in April 2009. Thus it is known as the pandemic, H1N1/09 virus by the WHO. The symptoms of H1N1 flu are similar to those of other influenzas, and may include fever, cough (typically a dry cough), headache, muscle or joint pain, sore throat, chills fatigue running nose, diarrhoea and neurological problems, etc.
People at higher risk of serious complications includes those aged over 65, children younger than 5, children with neurodevelopmental conditions, pregnant women, etc.
33. In angiosperms, the microsporangia develops to form
(a) stigma
(b) ovule
(c) endosperm
(d) pollen sacs
Ans. (d)
Sol. A microsporangium is a sporangium that produces spores that gives rise to a male gametophyte in gymnosperms and angiosperms. The microsporangia produces the microsporocyte, also known as the microspore mother cell, which then creates four microspores through meiosis, These microspores divide through mitosis to create pollen grains. A very young anther consists of an actively dividing meristematic cells surrounded by a layer of epidermis. If then becomes two lobed. Each anther lobe develops four pollen sacs. Thus, a two-lobed anther develops four pollen sacs, situated at four corners of the anther.
34. Given the pKa values of different acidic sites in cysteine, the principal ionic form in which it exists at pH 7.0, is
(a) 
(b) 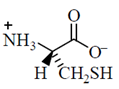
(c) 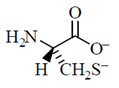
(d) 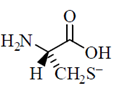
Ans. (b)
Sol.
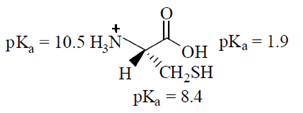
Since, its ionic form exist at pH 7.0, i.e. neutral form. It is possible by zwitter ion formation.
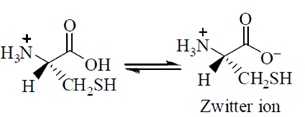
35. In .................. evolution, ...................... anatomical structures develop in different directions to adapt different functions
(a) convergent, homologous
(b) divergent, homologous
(c) convergent, analogous
(d) divergent, analogous
Ans. (b)
Sol. Divergent evolution is the accumulation of difference between groups which can lead to the formation of new species, usually a result of diffusion of the same species to different and isolated environment which blocks the gene flow among distinct populations, allowing differentiated fixation of characteristics. Divergent evolution is the result of homologous anatomical structures which develop in different directions in order to adapt different functions.
36. A model of gene control for the Iac operon is shown below
Match the component of Iac operon in Group I with the function listed in Group II
Group I Group II
K. O 1. Encodes protein -galactoside permease
L. P 2. Provides binding site for RNA polymerase
M. Y 3. Initiates lac mRNA synthesis
N. A 4. Encodes protein thiogalactoside transacetylase
(a) K–2, L–3, M–4, N–1
(b) K–3, L–2, M–4, N–1
(c) K–3, L–2, M–1, N–4
(d) K–2, L–3, M–1, N–4
Ans. (c)
Sol. Lac operon is an operon required for the transport and metabolism of lactose in E.coli and many other enteric bacteria.
Operator, in the lac operon is meant to initiate the process of mRNA synthesis. Promotor, is the region of the lac operon which provides a binding site for the RNA polymerase. Lac Y, encodes for the protein 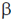 -galactosidase permease, a transmembrane symporter that pumps
-galactosidase permease, a transmembrane symporter that pumps 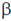 -galactosides into the cell using a protein gradient in the same direction.
-galactosides into the cell using a protein gradient in the same direction.
Lac A, encodes protein thiogalactoside transacetylase which transfers an acetyl group from acetyl Co-A to 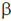 -galactosides.
-galactosides.
37. Venkatraman Ramakrishan was awarded noble prize in 2009 in chemistry for studying the structure and functions of
(a) ribosome
(b) nulceosome
(c) splicesome
(d) graphine
Ans. (a)
Sol. Venkataraman Ramakrishnan is an Indian born American and British structural biologist. In 2009 he shared the Nobel Prize in chemistry with Thomas A. Steilh andAda Yednath, for the studies of the structure and function of the ribosome. He begain his work on ribosomes as a postadoctral fellow with Peter Moove at Yale University. In 2005, Ramakrishanan's labratory published a 5.5 Angstrom resolution structure of the 30S subunit. The following year, his laboratory determined the complete molecular structure of the 30S subunit of ribosome and its complexes with several antibiotics.
38. During replication helicase enzyme separates paraental strands of DNA in physiological conditions.
In a Polymerase Chain Reaction, the function of helicase is achieved by
(a) substrate level phosphorylation
(b) oxidative phosphorylation
(c) dehydrogenation
(d) isomerization
Ans. (b)
Sol. Oxidative phosphorylation is the metabolic pathway in which the mitochondria in cells uses their structure, enzymes and energy released by the oxidation of nutrients to reform ATP.
39. During replication helicase enzyme separates parental strands of DNA in physiological conditions.
In a Polymerase Chain Reaction, the function of helicase is achieved by
(a) taq polymerase
(b) high temperature
(c) primase
(d) Mg++ ions
Ans. (d)
Sol. MgCl2 acts as a cofactor for Taq enzyme acting as a catalyst. Thus, during the PCR reaction, d NTP's are dissociated into dNMPs to form phosphodiester bond between 3' OH of adjacent nucleotide and 5' phosphate of the upcoming group of d NTP and helps in the removal of the beta and gamma phosphate from d NTP. It has been reported that incredasing the concentration of Mg2+ increases the activity of Taq DNA polymerase but at the derviation of specificity. On the other hand, the lesser concenration of Mg2+ will lower the Taq's activity but increases its specificity. Hence, It can be said that the role played by helicase in replication is similar to the role played by Mg2+ in PCR.
40. In cats, white skin is dominant over grey, black eye is dominant over grey, and curl tail is dominant over straight. A cat homozygous for white skin, grey eye, curl tail mates with another cat homozygous for white skin, black eye, straight tail. What percentage of F1 generation will have white skin, black eye, curl tail phenotype?
(a) 25%
(b) 100%
(c) 50%
(d) 75%
Ans. (b)
Sol. In the given question, we have the following alleles in cats
White skin : WW (Dominant)
Grey skin : ww (Recessive)
Black eye : BB (Dominant)
Grey eye : bb (Recessive)
Curl tail : CC (Dominant)
Straight tail : cc (Recessive)
If we cross a homozygous for white skin, grey, eye, curl tail i.e., WWbbCC with homozygous for white skin, black eye, straight tail i.e. WWBBcc, we get 100% F1 generation with white skin, black eye, curl tail.
41. Which given pair of greenhouse gases has highest contribution towards global warming?
(a) CO2 and CH4
(b) CO2 and CFC
(c) CO2 and N2O
(d) CFC and CH4
Ans. (a)
Sol. A green house gas in any gaseous compound in the atmosphere that is capable of absorbing infrared radiation, thereby trapping and holding that in the atmosphere. By increasing the heat in the atmosphere, green house gases are responsible for the green house effect which ultimately leads to global warming. Carbon dioxide (CO2) and methane (CH4) are the two gases that contribute most towards the global warming.
42. The INCORRECT statement regarding second messenger, adenosine 3', 5'-cyclic nucleotide monophosphate (cAMP) is
(a) it acts as a second messenger for many regulatory molecules
(b) it acts as an intracellular second messenger in neurons
(c) it activates specific cyclic nucleotide dependent protein kinases
(d) it provides source of energy for cells
Ans. (d)
Sol. Cyclic AMP or (cAMP) is an important second messenger in many biological processes. It is formed when the membrane enzyme, adenyl cyclase, becomes activated. This cAMP then goes to activate other specific proteins. cAMP is derived from ATP and is mainly used in signal transduction in many different organisms, such as transfering into cells the effects of hormones, etc. Conveying the cAMP-dependent pathway. It also acts as an intracelular second messenger in neurons and activates specific cyclic nucleotide dependent protein kinase. cAMP does not act as a source of energy for cells.
43. In lactic acid fermentation, lactate dehydrogenase gene becomes non-functional due to mutation. The product that will accumulate at the end of this process is
(a) pyruvate
(b) lactic acid
(c) acetaldehyde
(d) ethyl alcohol
Ans. (a)
Sol. Lactic acid fermentation is a biological process by which glucose and other six carbon sugars are converted into cellular energy and them metabolite lactage. Pyruvate acts as the terminal electron acceptor in lactic acid fermentation. When sufficient oxygen is not present in the muscle cells for further oxidation of pyruvate and NADH produced in glycolysis, NAD+ is generated from NADH by reduction of pyruvate to lactic acid. This conversion is done by the action of an enzyme lactate dehydrogenase.
44. The deficiency of vitamin A in human leads to
(a) sterility
(b) rickets
(c) night blindness
(d) scuvy
Ans. (c)
Sol. Night blindness or nyctalopia is the inability to see well at night or in the poor light. It is not regaded as a disease in itself but is rather a symptom of an underlying problem of several eye diseases. It may exist from birth or may be caused due to and injury or due to the deficiency of Vitamin-A in the diet. The most common cause of nyctalopia is retinitis pigmentosa, a disorder in which the rod cells in the retina gradually lose their ability to respond to the light.
45. 2-Butyne can be selectively reduced to trans-2-butene using
(a) H2, Pd/C
(b) H2, Pd/CaCO3, quinoline
(c) LiAlH4
(d) Na/liq. NH3
Ans. (d)
Sol.
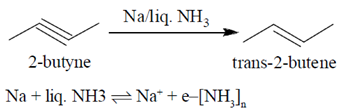
Electrons are very strong reducing agent due to its small size and high energy. 2-butyne can be selectively reduced to trans-2-butene using Na/liq. NH3 because odd electrons are present anti to each other.
46. The correct Fischer projection reperesentation of the following compound, is
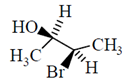
(a) 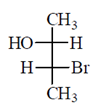
(b) 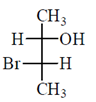
(c) 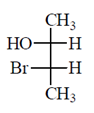
(d) 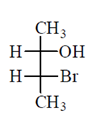
Ans. (d)
Sol.
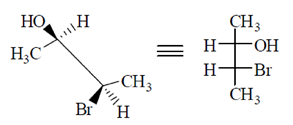
All the molecule which are present above the plane arel lie right hand side in Fischer projection representation.
47. Match the compounds in Group I wit their appropriate spectroscopic data in Group Ii.
Group I
P. CH3COCH3
Q. CH3CH2COH
R. CH3COOCH3
Group II
1. two singlets of equal intensity in the 1H-NMR spectrum
2. a band at 1720 cm–1 in the IR spectrum
3. an intense peak at m/z 45 in the mass spectrum
(a) P–1, Q–2, R–3
(b) P–2, Q–3, R–1
(c) P–1, Q–3, R–2
(d) P–2, Q–1, R–3
Ans. (a)
Sol.
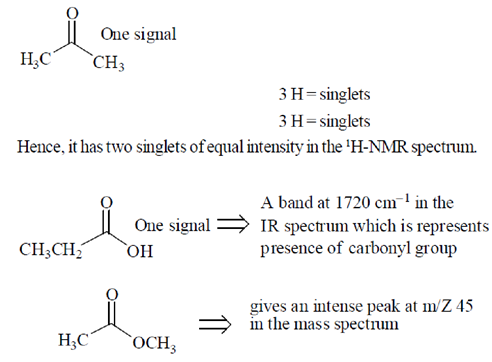
48. Among the following compounds, the one that is soluble in aqueous NaOH but not in aqueous NaHCO3, is
(a) 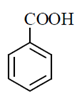
(b) 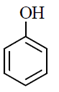
(c) 
(d) 
Ans. (b)
Sol.
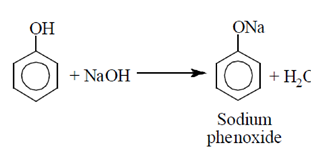
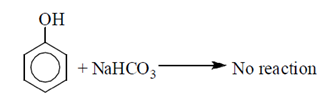
Phenol is weaker acid than carboxylic acid. Therefore, phenol does not react with NaHCO3.
The acidic character of phenol is because of the presence of polar —OH group. The —OH group in phenol is directly attached to the sp2 hybridised carbon of benzene ring which acts as an electron withdrawing group.
49. The major product of the following reaction sequence, is
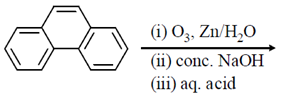
(a) 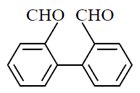
(b) 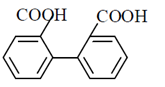
(c) 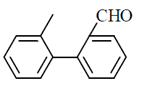
(d) 
Ans. (d)
Sol.
50. The major product formed in the E-2 elimination reaction of the following compound is
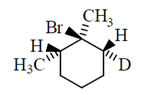
(a) 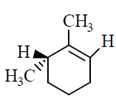
(b) 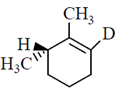
(c) 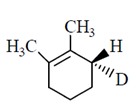
(d) 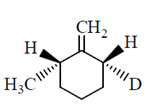
Ans. (a)
Sol.
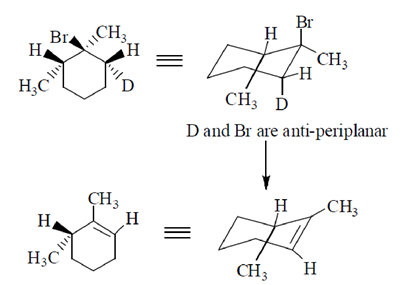
51. The most reactive diene towards Diels-Alder reaction, among the following is
(a) 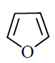
(b) 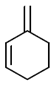
(c) 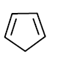
(d) 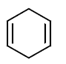
Ans. (c)
Sol. The most reactive diene towards Diels-Alder reaction is cisoid form of diene. Trans-form does not give effective reaction. The diene is the most reactive and these diene is HOMO and dienophile is LUMO. In Diels-Alder reaction, endo product is the major product due to secondary interaction. Therefore,  is the most reactive towards Diels-Alder reaction.
is the most reactive towards Diels-Alder reaction.
52. The correct structure of pyrophosphorus acid is
(a) 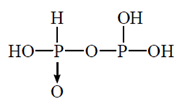
(b) 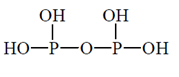
(c) 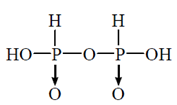
(d) 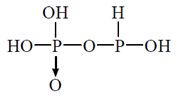
Ans. (c)
Sol. Pyrophosphorus acid contains P in the oxidation state (+III), and which are reducing agents.
P is four coordinate and tetrahedrally surrounded wherever possible.  back bonding gives rise to p=O bond. The hydrogen atoms in —OH groups are ionisable and are acidic, but the P—H bonds in the phosphorus acid have reducing, not acidic, properties.
back bonding gives rise to p=O bond. The hydrogen atoms in —OH groups are ionisable and are acidic, but the P—H bonds in the phosphorus acid have reducing, not acidic, properties.
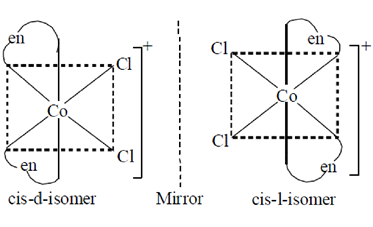
53. Among the following complexes, the one which exhibits optical isomerism is
(note : en = ethylenediamine)
(a) cis-[Co(en)2Cl2]+
(b) cis-[Pt(NH3)2Cl2]
(c) trans-[Co(en)2Cl2]+
(d) trans-[Pt(NH3)2Cl2]
Ans. (a)
Sol. cis[Co(en)2Cl2]+ complex ion exists cis and trans-isomers, cis-isomers is chiral and optically active.
54. The gas that is produced on treating NaCl with conc. H2SO4 is
(a) O2
(b) Cl2
(c) SO2
(d) HCl
Ans. (d)
Sol. Conc. H2SO4 reacts with solid NaCl in the cold to produced hydrogen chloride and sodium hydrogen chloride and sodium hydrogen sulphate.
NaCl + conc. H2SO4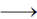 HCl + NaHSO4
HCl + NaHSO4
55. The correct order of the atoms in terms of their first ionization energy is
(a) Li < B < Be < C
(b) Li < Be < B < C
(c) Li > B > Be > C
(d) Li > Be > B > C
Ans. (a)
Sol. The general trend of first ionisation energy is an we move left to righ in period in the periodic table, size of atom decreases and then ionisation energy increases. But in case of Be and B, Be has higher first ionisation energy than B because electrons of Be arfe present in 2s orbital and B in 2p orbital. 2s orbital is strongly held by nucleus than 2p so, the IE of Be is stronger than B. Therefore, correct order will be Li < B < Be < C
56. The compound with square planar geometry is
(a) [Ni(CO)4]
(b) [NI(CN)4]2–
(c) [Ni(PPh3)2Cl2]
(d) [NiCl4]2–
Ans. (b)
Sol. [Ni(CN)4]2– forms square planar complex because the ligand CN– is the strong field ligand and period up the electron and form dsp2 hybrid orbital.
57. Match the molecules in Group I with their shape in Group II
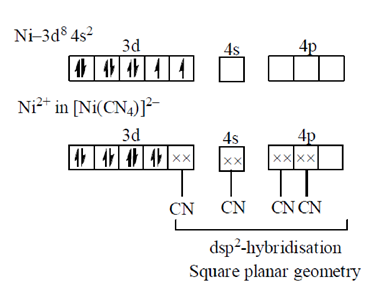
Group I Group II
P. ICl2– 1. trigonal bipyramid
Q. H2O 2. linear
R. PCl5 3. V-shaped
4. square pyramid
(a) P–3, Q–2, R–4
(b) P–4, Q–3, R–1
(c) P–2, Q–3, R–1
(d) P–4, Q–3, R–2
Ans. (c)
Sol.
Hence, the correct match is
P-2, Q-3, R-1
58. The spin-only magnetic moment of [Fe(CN)6]4– is
(a) 4.9D
(b) 0D
(c) 5.92D
(d) 2.82D
Ans. (b)
Sol. [Fe(CN)6]4–
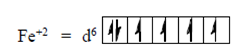
But in this complex ion, a strong field ligand (CN) is present which paired the all unpaired electrons, i.e.,
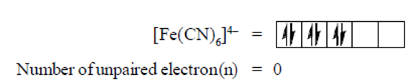
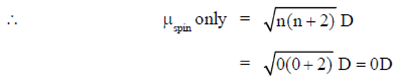
59. One mole of a gas absorbs 40J of heat. If the work done on the surrounding by the gas is 20J, then 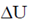 (in J) for the gas is
(in J) for the gas is
(a) 60
(b) 20
(c) –20
(d) –60
Ans. (b)
Sol. 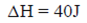

60. For the reaction, N2O4(g)  2NO2(g), taking place in a closed container at a constant tempertaure, the rate constant k in terms of P0(pressure at time t = 0) and Pt(pressure at time t) is given by
2NO2(g), taking place in a closed container at a constant tempertaure, the rate constant k in terms of P0(pressure at time t = 0) and Pt(pressure at time t) is given by
(a) 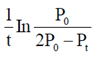
(b) 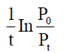
(c) 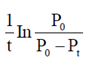
(d) 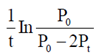
Ans. (a)
Sol. For the reaction,
N2O4(g) 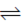 2NO2 (g)
2NO2 (g)
Initial pressure p0 0
At equilibrium p0 – p 2p
Total pressure (pt) = p0 – p + 2p = p0 + p
or p = pt – p0
 The given reaction is first order
The given reaction is first order
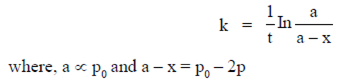
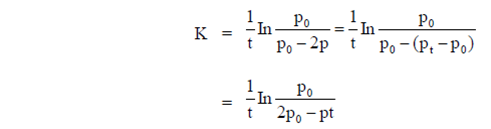
61. pKa
of acetic acid is 4.80. A 10 mL of 1M solution of acetic acid is mixed with 5mL of 1M solution of NaOH. The pH of the resulting solution is
(a) 3.2
(b) 7.0
(c) 4.8
(d) 2.4
Ans. (c)
Sol. NaOH + CH3COOH  CH3COONa + H2O
CH3COONa + H2O
Millimole before reaction 5 10 0 0
Millimole after reaction 0 5 5 5
The solution consists CH3COOH and CH3COONa and thus, is a buffer solution.
Hence, pH is given as,
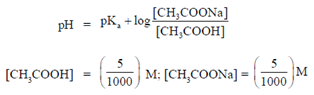
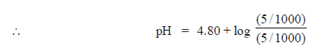
or pH = 4.80
Note Number or millimole = Molarity × Volume in mL.
62. The series that corresponds to transition from higher levels to n = 4 in the hydrogen spectrum is
(a) Paschen
(b) Balmer
(c) Pfund
(d) Brackett
Ans. (d)
Sol. n1 = 1, n2 = 2, 3 .............. Lyman series
n1 = 2, n2 = 3, 4 ............... Balmer series
n1 = 3, n2 = 4, 5 .............. Paschen series
n1 = 4, n2 = 5, 6 ............... brackett series
n1 = 5, n2 = 6, 7 ............... Pfund series
The H2 molecules are dissociated and the energetically excited H-atoms that are produced a spectrum of a series of 'lines'.
So. Brackett series, corresponds to transition from higher level n2 = 5, 6 ............... to lower level n1 = 4.
63. For the reaction, A→
product, match the order of the reaction in Group I with their corresponding linear plots in Group II
Group I Group II
P. Zero 1. In[A] versus time
Q. First 2. 1/[A] versus time
R. Second 3. [A] versus time
(a) P–1, Q–2, R–3
(b) P–2, Q–1, R–3
(c) P–3, Q–1, R–2
(d) P–1, Q–3, R–2
Ans. (c)
Sol. A 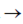 Product
Product
P. Zero order reaction
[A] = [A]0 – kt
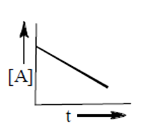
Plot [V] versus t will be straight line with negative slope.
Q. First order reaction
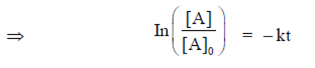
In plot of In [A] versus t, straight line is obtained having negative slope.
R. Second order reaction,
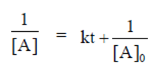
Plot versus t gives a straight line with positive slope.
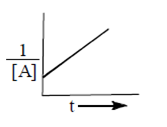
R. Second order reaction,
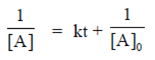
Therefore, correct, match will be
P-3, Q-1, R-2
64. 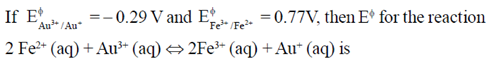
(a) +1.06V
(b) –1.06V
(c) –0.48V
(d) –1.83V
Ans. (b)
Sol.
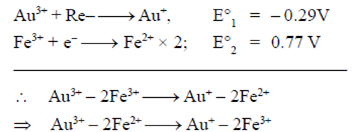
As, E° is an intensive property, i.e., non-additive.
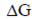 = ΔG1 – ΔG2
= ΔG1 – ΔG2
–nFE° = –nFE°1 + nFE°2
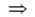 –E° = –E°1 + E°2
–E° = –E°1 + E°2
E° = E°1 – E°2
= –0.29 – 0.77 = –1.06 V
65. The depth of a swimming pool filled with clean water (refractive index = 4/3) appears to be 3m to a person standing near it. Its actual depth is
(a) 2.25m
(b) 4m
(c) 5.3m
(d) 9m
Ans. (b)
Sol. Actual depth = refractive index × apparent depth
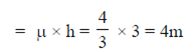
66. A semiconductor device that has two p-n junctions is
(a) rectifier-diode
(b) photo-diode
(c) transistor
(d) solar-cell
Ans. (c)
Sol. Transistor consists of two p-n junctions.
67. The resolution of a microscope is directly proportional to the wavelength of the radiation used for its operation. Among the following, maximum possible resolution can be achieved from
(a) optical microscope with blue light source
(b) optical microscope with yellow light source
(c) electron microscope operating at 100kV
(d) electron microscope operating at 200kV
Ans. (b)
Sol. We are given that the resolution of am icroscope is directly proportinoal to the wavelength of the radiation,
i.e., Resolution ∝ λ (wavelength)
As, 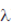 blue <
blue < 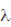 yellow yellow and both lie in the range (700nm to 400nm) of wavelength.
yellow yellow and both lie in the range (700nm to 400nm) of wavelength.
Now, for electron microscope operating at 100kV, we have
E = eV = e × 100 kV
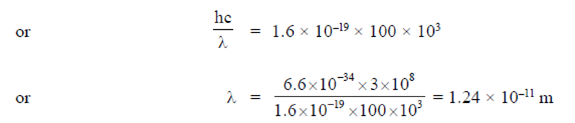
For electron microsvciope operating at 200 kV, we have
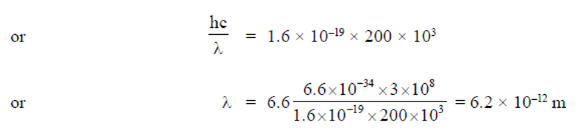
Hence, maximum possible resolution can be achieved optical microscope with yellow light source, as yellow light has maximum wavelength among the four.
68. Longitudinal waves can travel through
(a) gas only
(b) gas and liquid only
(c) gas and solid only
(d) gas, liquid and solid
Ans. (d)
Sol. Longitudinal wave can travel through any medium such as air (gas), water (liquid) or solid.
69. The waves, 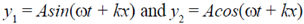
(a) are in same phase
(b) have a phase difference of 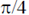
(c) have a phase difference of 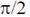
(d) have a phase difference of 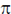
Ans. (c)
Sol. 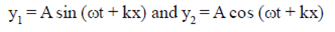
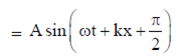
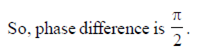
70. A vertical spring is fixed at its uper end. Same sized blocks of wood (W), glass (G) and copper (Cu) are attached to its lower end one at a time and the system is set into vertical oscillations.
The three measured time periods are in the order
(a) TCu > TG > TW
(b) TW > TG > TCu
(c) TG > TCu > TW
(d) TCu > TW > TG
Ans. (a)
Sol. As we know that time period of mass (M) suspended through any spring is given by
T = 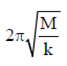
So, TCu > TG > TW (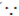 MCu > MG > MW)
MCu > MG > MW)
71. A neutron collides head-on with a He-atom at rest. Collision is elastic and He-atom recoil with a speed of 2 × 105 m/s. Then, the initial speed of the neutrons is
(a) 0.5 × 105 m/s
(b) 2 × 105 m/s
(c) 5 × 105 m/s
(d) 8 × 105 m/s
Ans. (c)
Sol. From the principle of conservation of momentum
2m1v1 = (m1 + m2)v2 (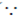 therfore m1 = 1, m2 = 4, v2 = 2 × 105)
therfore m1 = 1, m2 = 4, v2 = 2 × 105)
72. The two ends of a composite slab consisting of three layers of different thermal conductivities and different widths (as shown in figure) but same length and breadth are maintained at temperatures T1 and T2 (T1 > T2). Then the heat flow rate through
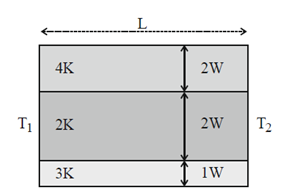
(a) all the three layers is same
(b) top layer is maximum
(c) middle layer is maximum
(d) bottom layer is maximum
Ans. (b)
Sol. Rate of flow of heat through the slab is given by
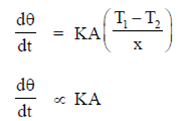
In case of top layer,  will be maximum for top layer (4K).
will be maximum for top layer (4K).
73. Match the actions is Group II that will produce radiations listed in Group I
Group I
P.  -rays
-rays
Q. UV radiation
R. IR radiation
Group II
1. H-atom in 1st excited state returns to ground state
2. A body at 600K emitting radiation
3. Fusion of two light nuclei
(a) P–3, Q–1, R–2
(b) P–3, Q–2, R–1
(c) P–1, Q–3, R–2
(d) P–1, Q–2, R–3
Ans. (a)
Sol. When H-atom in 1st excited state returns to ground state it produces UV-radiation.
Fusion of two light nuclei produces  -rays.
-rays.
A body at 600 K temperature emit infrated radiation.
74. A rigid conducting wire PQ is moving on conducting rails (as shown in figure) with constant speed v = 6m/s in a region of uniform field B = 0.2 Wb/m2. The magnitude of induced emf and direction of induced current are
(a) 1.8 V, clockwise
(b) 1.8 V, anti-clockwise
(c) 3.6 V, clockwise
(d) 3.6 V, anti-clockwise
Ans. (a)
Sol. Emf induced = lv × B
= lvB sin 90° = lvB = 1.5 × 6 × 0.2
= 1.8 V
Direction will be clockwise accrodin to Lenz law.
75. A ball is projected at 30° from ground with an initial velocity of 10 m/s. Taking g = 10 m/s2, the horizontal range of the ball is
(a) 2.5m
(b) 5m
(c) 8.66m
(d) 10m
Ans. (c)
Sol. As we know, horizontal range is given by
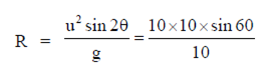
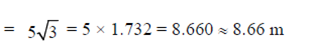
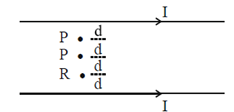
76. Consider equidistant points P, Q and R between two current carrying infinite straight parallel wires (as shown in figure) with current included magnetic fields ,  respectively. Then
respectively. Then
(a) 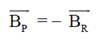
(b) 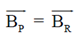
(c) 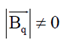
(d) 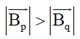
Ans. (a, d)
Sol. 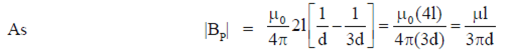

Also, BP = –BQ
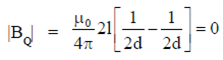
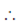 We can conclude that field at P and Q will be equal and opposite and also |BP| > |BQ|.
We can conclude that field at P and Q will be equal and opposite and also |BP| > |BQ|.
77. An object weights 50N on Earth (g = 10m/s2). Its mass on a planet having g = 2 m/s2 will be
(a) 1kg
(b) 2.5kg
(c) 5kg
(d) 10kg
Ans. (c)
Sol. On earth the mass of object will be 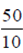 = 5 kg
= 5 kg
Since, mass does not change it remains constant, so on the other planet also its mass will be 5 kg.
78. An 80W fan, a 60W bulb and a 500W washing machine are operated for 15, 20 and 1hr, respectively. The total electrical power units consumed are
(a) 1.2
(b) 1.7
(c) 2.4
(d) 2.9
Ans. (d)
Sol. Total power consumed by fan in 15 hours
= 80 × 15
= 1200 Wh
Total power consumed by bulb = 60 × 20
= 1200 Wh
Total power consumed by 500 W washing machine
= 500 × 1 = 500 Wh
Total watt hour consumed = 1200 + 1200 + 500
= 2900 Wh
As 1 unit = 1 kWh
So, 2900 × 10–3 kWh = 2.9 units
79. An electron having a velocity 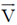 enters a region of uniform magnetic field
enters a region of uniform magnetic field 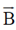 as shown in figure.
as shown in figure.
The effect of 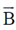 on the motion of electrons is that it will
on the motion of electrons is that it will

(a) continue to move without any deflection
(b) be reflected back
(c) be deflected up
(d) be deflected down
Ans. (a)
Sol. Force experienced by the charge placed in magnetic field is given by
F = qv × B = q |v| |B| sin 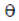
As 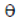 = 0
= 0
So, F = 0
80. In the given circuit, an ideal battery of 15 V and resistance of 4 ohm each are connected as shown below. The current (in amperes) through the ammeter A is
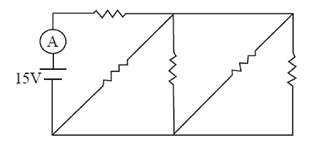
(a) 18.8
(b) 3
(c) 2.5
(d) 1.9
Ans. (b)
Sol. Circuit can be drawn as
So, from Ohm's law
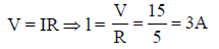
81. In a series LR circuit connected to an alternating source Vs, the measured voltage across L (ideal conductor) is 20V and across R is 15 V. The n the value of Vs is
(a) 20V
(b) 25V
(c) 25V
(d) 35V
Ans. (b)
Sol. From the series L-R circuit
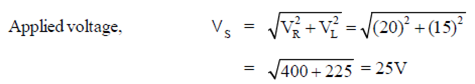
82. How does the electric field of a uniformly charged infinited metal sheet depend on the distance 'R' from the sheet?
(a) R–2
(b) R–1
(c) R–1/2
(d) Independent of R
Ans. (d)
Sol. Electric field of uniformly charged infinite metal sheet is given by, E = 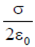 .
.
From the notation, it is clear that electric field is independent of distance R.
83. The value of 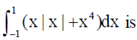
(a) 0
(b) 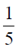
(c) 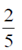
(d) 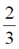
Ans. (c)
Sol. 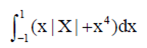
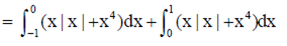
(by using property of defininte integral)
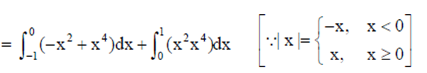
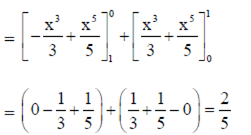
84. The radius of the circle x2 + y2 – 4x – 6y + 4 = 0 is
(a) 2
(b) 3
(c) 4
(d) 9
Ans. (b)
Sol. Given, equation of circle is,
x2 + y2 – 4x – 6y + 4 = 0
 (x2 – 4x + 4) – 4 + (y2 – 6y + 9) – 9 + 4 = 0
(x2 – 4x + 4) – 4 + (y2 – 6y + 9) – 9 + 4 = 0
 (x – 2)2 + (y – 3)2 = 9
(x – 2)2 + (y – 3)2 = 9 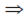 (x – 2)2 + (y – 3)2 = 32
(x – 2)2 + (y – 3)2 = 32
 Radius of the circle is 3 units.
Radius of the circle is 3 units.
85. For a complex number z, denotes its complex conjugate. Let z1 = x + iy and z2 = y + ix be two complex numbers such that |z1| = |z2| = 1. Then 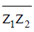 is equal to
is equal to
(a) 2xy-i
(b) 2xy
(c) –i
(d) i
Ans. (c)
Sol. Given, z1 = x + iy and z2 = y + ix
and |z1| = |z2| = 1
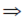 x2 + y2 = 1
x2 + y2 = 1
Now, z1z2 = (x + iy) (y + ix)
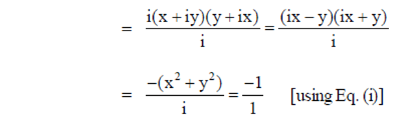
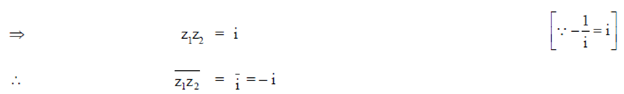
86. If 1 and 2 are roots of x2 + px + q = 0. 0, the p and q, respectively are
(a) –3 and 2
(b) 2 and –3
(c) 3 and –2
(d) –2 and 3
Ans. (a)
Sol. Given, 1 and 2 are the roots of x2 + px + q = 0
So, 1 and 2 satisfy the equation x2 + px + q = 0
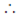 12 + p × 1 + q = 0 and 22 + p × 2 + q = 0
12 + p × 1 + q = 0 and 22 + p × 2 + q = 0
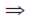 p + q = –1 ...(i)
p + q = –1 ...(i)
2p + q = –4 ...(ii)
Substract Eq. (ii) from Eq. (i), we have
–2p + p = 4 – 1  – p = 3
– p = 3
 p = –3
p = –3
Put p = – 3 in Eq. (i), we get
–3 + q = – 1  q = –1 + 3 = 2
q = –1 + 3 = 2
 p = – 3 and q = 2
p = – 3 and q = 2
87. The area of the region lying in the first quadrant bounded by the curve y2 = 4x and the line x = 2 is
(a) 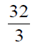
(b) 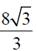
(c) 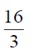
(d) 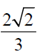
Ans. (b)
Sol. Given, y2 = 4x
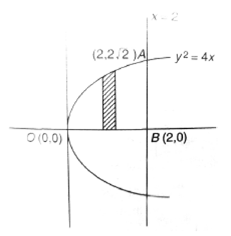
On solving y2 = 4x and x = 2, we have
Coordinates of A as 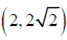
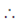 Required area in first quadrant
Required area in first quadrant

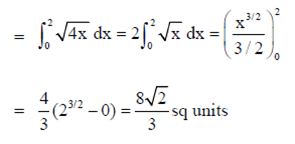
88. Let 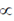 and
and 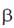 be two real numbers. If a matrix
be two real numbers. If a matrix 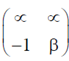 is symmetric and non-invertible, then
is symmetric and non-invertible, then 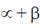 is equal to
is equal to
(a) 2
(b) 1
(c) 0
(d) –2
Ans. (d)
Sol. Let A = 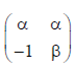
Given, A is symmetric
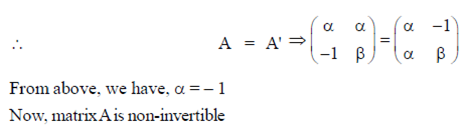

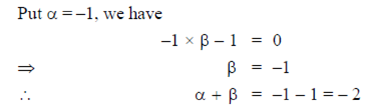
89. If the sum of the infinite series 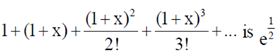 then x is
then x is
(a) 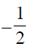
(b) 0
(c) 1
(d) 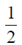
Ans. (a)
Sol. We know that
ey = 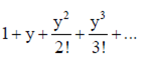
= e1/2 (given, sum = e1/2)
 e1 + x = e1/2
e1 + x = e1/2
On comparing exponents, we have
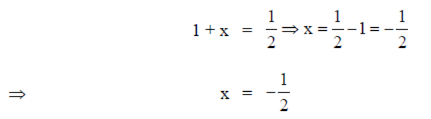
90. The minimum value of the function f(x) = x4 – 2x + 2 in [–1, 2] is
(a) 1
(b) 2
(c) 0
(d) –2
Ans. (a)
Sol. Given function is
f(x) = x4 – 2x2 + 2 in [–1, 2] ...(i)
Now, f'(x) = 4x3 – 2 × 2x + 0
f'(x) = 4x3 – 4x
f'(x) = 4x(x2 – 1)
For minimum value f' (x) = 0
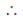 4x(x2 – 1) = 0
4x(x2 – 1) = 0
x(x2 – 1) = 0 
x = 0
and x2 – 1 = 0
 x2 = 1
x2 = 1
x = 0
and x = ± 1
But x  [–1, 2]
[–1, 2]
 x = 1
x = 1
f(1) = (1)4 – 2(1)2 + 2
= 1 – 2 + 2 = 1 [ from Eq. (i)]
from Eq. (i)]
Hence, the required minimum value of f(x) is 1.
91. Two ants P and Q are initially at a distance 148 m apart. They decide to meet. At the end of the first day, P covers a distance of 10m towards Q while Q covers a distance of 5m towards P. On each subsequent day, the distance covered by P reduces by 1m and than by Q increases by 2m of the previous day. The two ants will meet at the end of
(a) 9th day
(b) 8th day
(c) 7th day
(d) 6th day
Ans. (b)
Sol. 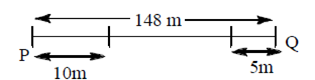
Given, on each subsequent day, the distance covered by P reduces by 1 m.
 We have a series
We have a series
10 + (10 – 1) + (10 – 2) + (10 – 3) + ... which is in AP
Let total number of terms is n and sum is x
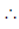 x =
x = 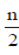 [2 × 10 + (n – 1) (–1)] ...(i)
[2 × 10 + (n – 1) (–1)] ...(i)
Now, distance covered by Q is increases by 2 m per day.
So, we have a series
5 + (5 + 2) + (5 + 2 × 2) + ... which is in AP.
If we take number of terms as n, then sum is 148 – x.
 148 – x =
148 – x = 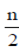 [2 × 5 + (n – 1) 2] ...(ii)
[2 × 5 + (n – 1) 2] ...(ii)
Adding Eqs. (i) and (ii), we have
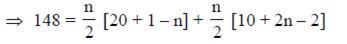
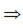 296 = 21n – n2 + 8n + 2n2
296 = 21n – n2 + 8n + 2n2
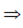 n2 + 29n – 296 = 0
n2 + 29n – 296 = 0
 n2 + 37n – 8n – 296 = 0
n2 + 37n – 8n – 296 = 0
 n(n + 37) – 8(n + 37) = 0
n(n + 37) – 8(n + 37) = 0
 (n + 37) (n – 8) = 0
(n + 37) (n – 8) = 0
 n = –37, 8 (negative not possible)
n = –37, 8 (negative not possible)
So, n = 8
 Two ants meet each other at the end of 8th day.
Two ants meet each other at the end of 8th day.
92. The equation of the line that makes an intercept of 2 with x-axis and is perpendicular to the line x + y – 1 = 0 is
(a) x + y – 1 = 0
(b) x + y + 2 = 0
(c) x – y – 2 = 0
(d) x – y + 2 = 0
Ans. (c)
Sol. Given, x + y – 1 = 0
y = –x + 1 ...(i)
On comporing eqn (i) with y = mx + c, we get
m = –1 ...(ii)
New, slope of the live perpendicular to x + y – = 0 is m1
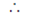 m1 m = –1
m1 m = –1
m1 × –1 = –1 [ from eq (ii)]
from eq (ii)]
 Equation of the line flat maeks an intercept Q with x-axis and having slope m1 = 1 is
Equation of the line flat maeks an intercept Q with x-axis and having slope m1 = 1 is
y – 0 = 1 (x – 2)
y = x – 2
x – y – 2 = 0
Hence the required equation is x – y – 2 = 0
93. 3 Mathematics, 2 Physics and 2 Chemistry books, all 7 by different authors, are to be arranged on a book shelf such that all the books of the same subject are together on the shelf. The total number of possible arrangements is
(a) 5040
(b) 720
(c) 144
(d) 24
Ans. (c)
Sol. Number of Mathematics books = 3
Number of Physics books = 2
Number of Chemistry books = 2
 All the seven books are written by different authors.
All the seven books are written by different authors.
Then, the total number of possible arrangement, when all the books of the same subject are together on the shelf
= (3! × 2! × 2!) × 3! = 144
94. If the point (1, 0, 1) is one extremity of the diameter of the sphere
x2 + y2 + z2 + 2x – 4y + 2z – 6 = 0
then its other extremity is
(a) (1, 4, 1)
(b) (–3, 0, –3)
(c) (3, –4, 3)
(d) (–3, 4, –3)
Ans. (d)
Sol. Equation of sphere is
x2 + y2 + z2 + 2x – 4y + 2z – 6 = 0
 (x2 + 2x + 1) + (y2 – 4y + 4) + (z2 + 2z + 1) – 6 – 6 = 0
(x2 + 2x + 1) + (y2 – 4y + 4) + (z2 + 2z + 1) – 6 – 6 = 0
 (x + 1)2 + (y – 2)2 + (z + 1)2 = 12 ...(i)
(x + 1)2 + (y – 2)2 + (z + 1)2 = 12 ...(i)
 Centre of the sphere is (–1, 2, – 1)
Centre of the sphere is (–1, 2, – 1)
 Point (1, 0, 1) is one extremity of the diameter.
Point (1, 0, 1) is one extremity of the diameter.
So, the equation of line passing through (1, 0, 1) and centre (–1, 2, – 1) is
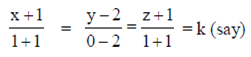
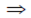 x = 2k – 1, y = –2k + 2, z = 2k – 1 ...(i)
x = 2k – 1, y = –2k + 2, z = 2k – 1 ...(i)
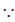 Above equation of line gives the diameter of sphere.
Above equation of line gives the diameter of sphere.
So, it satisfy the equation of sphere.
So, (2k – 1 + 1)2 + (2 – 2k – 2)2 + (2k – 1 + 1)2 = 12 [from Eq. (i)]
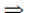 4k2 + 4k2 + 4k2 = 12
4k2 + 4k2 + 4k2 = 12
 k2 = 1
k2 = 1
 k = ± 1
k = ± 1
On putting k = 1 in Eq. (ii), gives
x = 1, y = 0 and z = 1
which is one extremity of the diameter.
Further put k = –1, in Eq. (ii), gives
x = –3, y = 4
and z = –3
 (–3, 4, –3) is second extremity of the diameter of the sphere.
(–3, 4, –3) is second extremity of the diameter of the sphere.
95. Let f be the function defined for real x as 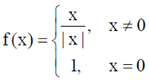 . Then f is
. Then f is
(a) continuous for all real x
(b) right continuous at x = 0
(c) a non negative function for all real x
(d) left continuous at x = 0
Ans. (b)
Sol. Given function is
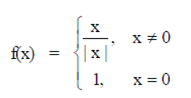
To check continuity of above function, we find left hand limit and right hand limit at x 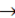 0.
0.
LHL = 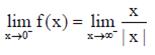

RHL = 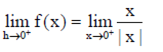

and value of the function at x = 0 is
f(0) = 1
From Eqs. (i), (ii) and (iii), we have
LHL 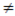 RHL
RHL
and RHL = f(0)
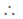 f(x) is right continuous at x = 0
f(x) is right continuous at x = 0
96. An urn consists of 10 items out of which 4 are defective. Three items are chosen randomly from the urn. The probability that exactly 2 from the chosen items are defective, is
(a) 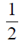
(b) 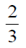
(c) 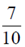
(d) 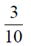
Ans. (d)
Sol. Since, urn consists of 10 items out of which 4 are defective
 When three items are chosen, then the required probability that exactly 2 are defective
When three items are chosen, then the required probability that exactly 2 are defective
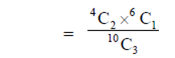
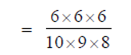
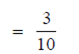
97. The eccentricity of the ellipse 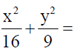
(a) 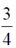
(b) 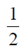
(c) 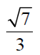
(d) 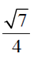
Ans. (d)
Sol. Given equation of ellipse is
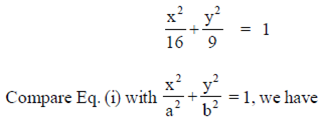
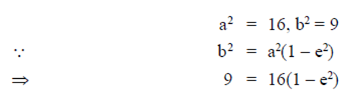

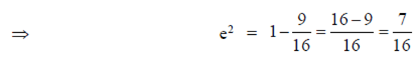
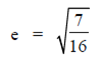
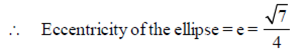
98. Suppose the statement
"If the flower smells sweet then I will buy it", is given to be FALSE. Then which one of the following is correct.
(a) The flower does not smell sweet and I bought it
(b) The flower does not smell sweet and I did not buy it
(c) The flower smells sweet and I bought it
(d) The flower smells sweet and I did not buy it
Ans. (b)
Sol. The negative of given statement is
"The flower does not smell sweet and I did not but it."
99. The values obtained in 20 throws of a die are given in the following frequency table
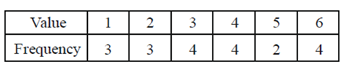
The sample median is
(a) 3
(b) 3.5
(c) 4
(d) 4.5
Ans. (b)
Sol. Given,
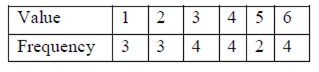
Sum of frequency = 3 + 3 + 4 + 4 + 2 + 4 = 20

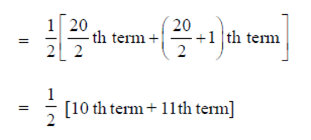
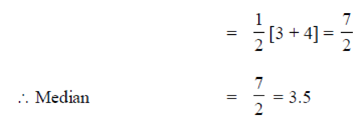
100. The equation of the normal to the curve x2y2 = 4 at the point (2, 1) is
(a) y = 3x – 5
(b) 5y = 3x – 1
(c) 3y = 5 – x
(d) 5y = –x + 7
Ans. (a)
Sol. Given equation of curve is
x2y3 = 4
Differentiate Eq. (i) with respect to x, we have
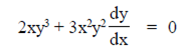
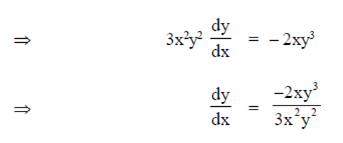
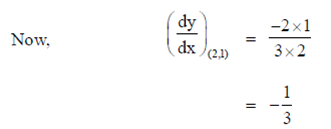
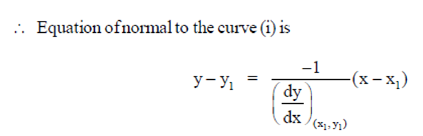
where (x1, y1) = (2, 1)
 y – 1 =
y – 1 = 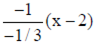
 y – 1 = 3(x – 2)
y – 1 = 3(x – 2)
 y – 1 = 3x – 6
y – 1 = 3x – 6
 y = 3x – 5
y = 3x – 5
