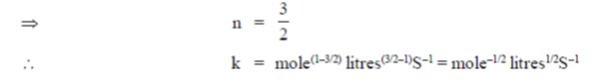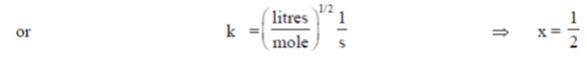IIT JAM Biology 2010
Previous Year Question Paper with Solution.
1. The composition of proteins P1 and P4 are shown below :
Protein Composition
P1 Rich in polar residues; poor in apolar residues
P2 Rich in apolar residues; poor in polar residues
P3 Has comparable number of polar and apolar residues
P4 Rich in glycine and proline
Which one of the following options CORRECTLY relates the propensities of these proteins to be folded, aggregated or disordered in an aqueous bffered solution?
(a) P1, P2 and P4 are disordered and P3 is folded
(b) P1 and P3 are folded, P2 is aggregated and P4 is disorderd
(c) P1 and P3 are folded, and P2 and P4 are disordered
(d) P1 and P4 are disorderd, P2 aggregated and P3 is folded
Ans. (b)
Sol. P1 and P3 are folded because they are rich in polar residues, i.e., hydrophilic amino acids. It has been observed that all polar groups are capable of forming side chain-side chain or side chain-main chain hydrogen bonds in proteins which in often very crucial for the stabilsation of the protein three-dimensional structure (i.e. folding). Polar or changed amino residues have high propensity to be in contact with polat solvent like water, due to their ability to form hydrogen bonds. P2 is aggregated because it is rich in non-polar residues, i.e., hydrophobic amino acids. Such residues build up the core and are not accessible to solvent and the polar surface in contact with the environment.
P4 is rich in glycine and proline due to which it considered as disordered protein. The disordered proteins show low sequence complexity and significant amino and compositional bias. Their constructured configurations are mainly due to their sequence biases. Glycine and proline are two particular residues that often appear in the sequence of such (disordered) proteins. Both of them contribute to disorder but for opposite reasons. Glycine, lacking any side chain, is so flexible that order is entropically unfavourable. Proline's cyclic side chain, by contrast, is stiff and therefore highly restricted in movement and mostly disturbing.
2. EnzP, EnzQ, EnzR and EnzS catalyze the metabolic reactions as shown below :
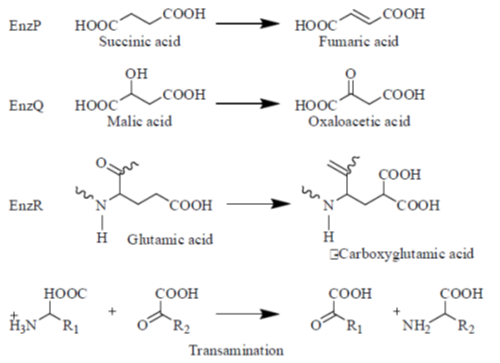
Each of the above enzymes is dependent on one of the following four vitamins (either the vitamin itself or its derivative) :
Vit B2 : Vitamin B2 (riboflavin)
Vit B3 : Vitamin B3 (niacin)
Vit B6 : Vitamin B6 (pyridoxal)
Vit K : Vitamin K
Which one of the following options gives the correct enzyme-vitamin matches?
(a) EnzP and Vit B3, EnzQ and Vit B2, EnzR and Vit B6, EnzS and Vit K
(b) EnzP and Vit B2, EnzQ and VIt B3, EnzR and Vit B6, EnzS and Vit K
(c) EnzP and Vit B2, EnzQ and Vit B3, EnzR and Vit K, EnzS and Vit B6
(d) EnzP and Vit B6, EnzQ and Vit B2, EnzR and VIt B3, EnzS and Vit K
Ans. (c)
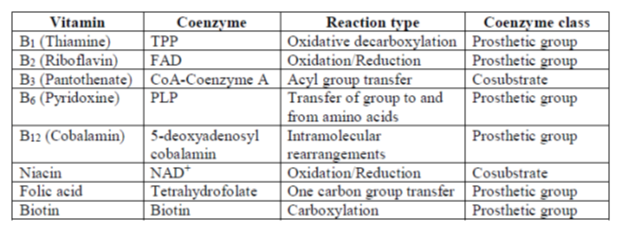
Sol. Many coenzymes are derived from vitamins. The given table shows the list of vitamins, the coenzymes derived from them, the type of reactions in which they participate, and the class of coenzyme.
Prosthetic groups are tightly bound to enzymes and participate in the catalytic cycles of enzymes.
VITAMINS AND COENZYMES
During the catalytic cycle of the enzyme succinate dehydrogenase (Enz-P), FAD (flavin adenine dinucleotide) is a prosthetic group that accepts two electrons from succinate, yielding fumarate as a product. FAD is derived from the vitamin B2 (Riboflavin).
In the second cataltic reaction, the molecule nicotinamide adenine dinucleotide (NAD) acts as a co-substrate in the oxidation-reudction reaction that is catalysed by enzyme malate dehydrogenase (Dnz- Q), one of the enzymes of the citric acid cycle. Here, NAD+ accepts two electrons from malic acid, yielding oxaloacetic acid. This NAD+ is derived from the vitamin B3 (Niacin).
In the third catalytic reaction, specific glutamate residues-are converted into Y-carboxyglutamic acid residues by a vitamin k-dependent carboxylase (Enz. R) in the presence of vitamin K. This (Vit K) is a fat soluble vitamin and an enzyme co-factor for post transcriptional, modification of a selected group of proteins.
In the fourth catalytic reaction, i.e., transamination reactions most standard amino lose their 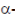 -amino group that is transferred to
-amino group that is transferred to 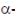 -keto group. This reaction is accomplished by enzymes called transaminases or aminotrasferaser (Enz-S). In such reactions, vitamin B6 or pyridoxine acts as a coenzyme.
-keto group. This reaction is accomplished by enzymes called transaminases or aminotrasferaser (Enz-S). In such reactions, vitamin B6 or pyridoxine acts as a coenzyme.
3. The ground state energy of hydrogen atom is –13.6 eV. Assume hc = 1240 eV, nm. The maximum wavelength in Balmer series (in nm) is approximately
(a) 103
(b) 122
(c) 244
(d) 653
Ans. (d)
Sol. For Balmer series, the wavelength is defined by the formula
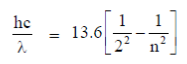
For maximum 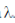 , electrons jump from 3rd to 2nd orbit
, electrons jump from 3rd to 2nd orbit
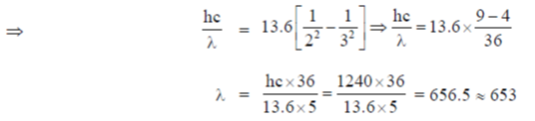
4. A sample of gas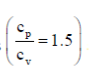 at a higher pressure P and temperature T, is suddenly released to atmosphere. The final temperature of the gas is T/2. The value of P (in the units of atm) is
at a higher pressure P and temperature T, is suddenly released to atmosphere. The final temperature of the gas is T/2. The value of P (in the units of atm) is
(a) 4
(b) 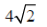
(c) 8
(d) 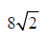
Ans. (c)
Sol. To find the value of p in atm
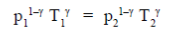
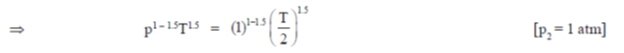
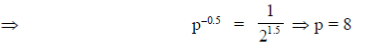
5. A ball is thrown with a speed of 40 m/sec in a direction of 30° with the ground. Assume g = 10m/sec2. The ball will reach to a maximum height (in meters) of
(a) 20
(b) 40
(c) 60
(d) 80
Ans. (a)
Sol. The maximum height attained by the ball is given by
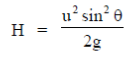
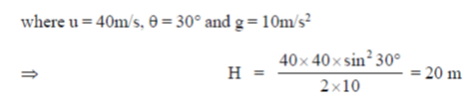
6. The dimensions ML2 T–2 do not correspond to
(a) work
(b) torque
(c) heat
(d) angular momentum
Ans. (d)
Sol. Dimension a of work = MLT–2 × L = ML2T–2 [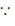 work = force × distance]
work = force × distance]
Dimensions of torque = MLT–2 × L = ML2T–2
[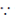 torque = force × perpendicular distance]
torque = force × perpendicular distance]
Dimensions of heat = ML2T–2 [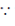 heat (energy) = work]
heat (energy) = work]
Dimensions of angular momentum = ML2 × T–1 = ML2T–1
[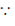 angular momentum = moment of interia × angular velocity]
angular momentum = moment of interia × angular velocity]
Thus, dimensions ML2T–2 do not correspond to angular momentum.
7. 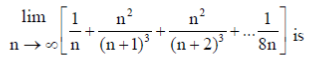
(a) 1/8
(b) ¼
(c) 3/8
(d) 1/2
Ans. (c)
Sol. 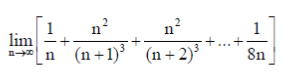
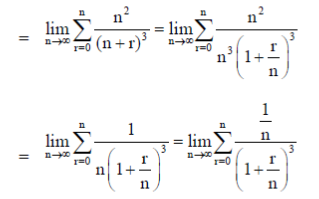
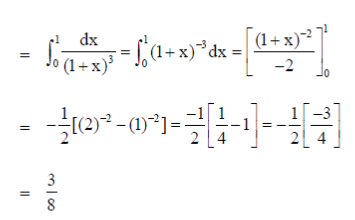
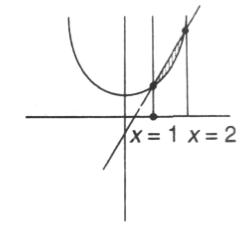
8. The area (in square units of length) enclosed by y = 2x2 + 1 and 6x – y = 3 is
(a) 1/3
(b) 2/3
(c) 1
(d) 4/3
Ans. (a)
Sol. Given curve are y = 2x2 + 1 and 6x – y = 3
Now, intersecting point of parabola and line is given by
6x – 3 = 2x2 + 1
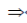 2x2 – 6x + 1 + 3 = 0
2x2 – 6x + 1 + 3 = 0
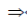 2x2 – 6x + 4 = 0
2x2 – 6x + 4 = 0
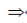 2(x2 – 3x + 2) = 0
2(x2 – 3x + 2) = 0
 x2 – 3x + 2 = 0
x2 – 3x + 2 = 0
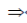 x2 – 2x – x + 2 = 0
x2 – 2x – x + 2 = 0
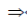 x(x – 2) –1(x – 2) = 0
x(x – 2) –1(x – 2) = 0
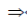 (x – 2)(x – 1) = 0
(x – 2)(x – 1) = 0
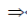 x = 1, 2
x = 1, 2
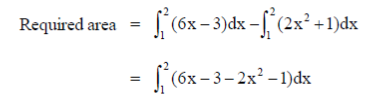
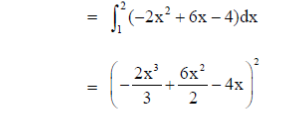
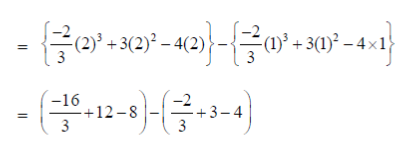
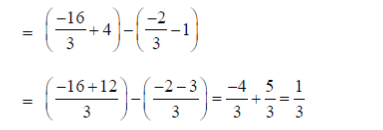
9. Suppose the principal increases continuously at the rate of r% per year. If Rs. 100 doubles in 10 years, then r is
(a) 5 loge2
(b) 10 loge2
(c) 50 loge2
(d) 100 loge2
Ans. (b)
Sol. Let P be the principal at any time t.
Since, principal increases continuously at the rate of r% 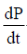 = r% of P
= r% of P
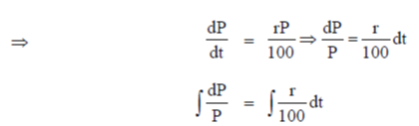
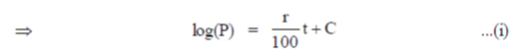
When t = 0 (initially) and let P = P0
log(P0) = 0 + C 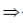 C = log(P0)
C = log(P0)
On substituting the value of C in Eq. (i), we get
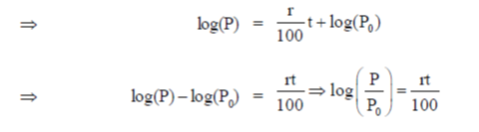
Given that. P0 = Rs. 100 and when t = 10, then P = 2P0
= Rs. 200
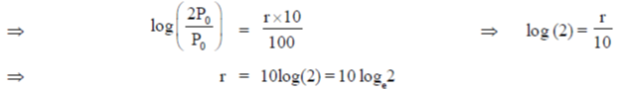
10. A eukaryotic cell lacking active telomerase
(a) will be unable to proofread incorrectly-added nucleotides
(b) is highly probable to be a cancerous cell
(c) will experience a gradual reduction of chromosome length with each, replication cycle
(d) will be unable to connect Okazaki fragments
Ans. (c)
Sol. A eukaryotic cell lacking active telomerase will experience a gradual reduction of chromosome length with each replication cycle. This is because telomere (Telomere terminal transferase) is a ribonucleoprotein that adds the nucleodide 'TTAGGG' to the 3' end of telomeres, which are found at the ends of eukaryotic chromosomes. It resembles reverse ranscriptases enzymes that synthesises. DNA using RNA template
11. Which of th following are used as reporter genes?
P.  -glucuronidase gene
-glucuronidase gene
Q. ampicillin-resistance gene
R. Gal4 gene
S. luciferase gene
(a) P and S
(b) P and Q
(c) R and S
(d) Q and R
Ans. (a)
Sol. Examples of reporter genes are luciferase (lux, luc) green fluorescent protein, 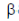 -glucuronidase, etc. A reporter gene is a gene that is used as an indication of whether a certain gene has been taken up by or expressed in the cell or organism population.
-glucuronidase, etc. A reporter gene is a gene that is used as an indication of whether a certain gene has been taken up by or expressed in the cell or organism population.
12. p-aminobenzoic acid is a biosynthetic precursor of
(a) glutamic acid
(b) acetic acid
(c) citric acid
(d) folic acid
Ans. (d)
Sol. p-aminobenzoic acid also known as p-aminobenzoic acid or PABA is an organic compound which is a biosynthetic precursor of folic acid. PABA is an intermediate in the synthesis of folate by bacteria, plants and fungi.
13. Ribosomes are made of
(a) DNA and proteins
(b) RNA and proteins
(c) only proteins
(d) DNA, RNA and proteins
Ans. (b)
Sol. Ribosomes consists of two major components, the small ribosomal subunit, which raeds the RNA and the large subunit, which joins amino acids to form a polypeptide chain. Each subunit is composed of one or more ribosomal RNA (r RNA) molecule and a variety of proteins.
14. The anticodon in tRNA is
(a) complementary to codon in rRNA
(b) complementary to codon in mRNA
(c) complementary to 3'-end of tRNA where amino acid binds
(d) changeable depending upon the amino acid it blinds to
Ans. (b)
Sol. The anticodon in a tRNA is complementary to codon in m RNA. The clover leaf structure of tRNA has a D-loop with 4 – 6 bp, variable loop, the acceptor stem 7 – 9 bp, a 5'-terminal anticodon arm 6 bp whose loop contain the anticodon complementary to m RNA.
15. Which one of the following hormones shows photoperiodicity?
(a) thyroxine
(b) melatonin
(c) cortisol
(d) relaxin
Ans. (b)
Sol. Melatonin is a hormone found in animals. It anticipates the daily onset of darkness. Becuase only in darkness, the key enzyme aralkylamine N-acetyltransferase is activated and converts serotonin to N-acetyl serotonin, which is ultimately converted to melatonin by final enzyme, acetyserotonin O-methyltransferase. Thus, we can say melatonin shows photoperiodicity.
16. Which of the following ligament(s) is/are attached to ovary?
P. ovarian ligaments
Q. suspensory ligaments
R. broad ligaments
(a) only P
(b) only P and Q
(c) only P and R
(d) P, Q and R
Ans. (d)
Sol. P, Q and R all are attached to the ovary as the ovarian. They cannot the ovary to the lateral surface of the uterus. The suspendory ligament of the ovary is a tissue that connnects the ovary to the wall of the pelvis. In the posterior region of the ovary the suspendory ligament is attached to the ovary via a continuous tissue called broad ligament.
17. The role of salicylic acid in systematic acquired resistance of plants is to
(a) directly destroy the pathogens
(b) activate defenses throughout the plant before the infection spreads
(c) activate heat shock proteins
(d) sacrifice the infected tissue
Ans. (b)
Sol. The role of salicyclic acid (SA) in systemic acquired resistance of plants is to activate defneses throughout the plant before the infection spreads. SA has long been known to play a central role in plant defnece against pathogenes. SA levels increase in plant tissue following pathognes infection and exogenous application of SA results in enhanced resistance to a broad range of pathogens.
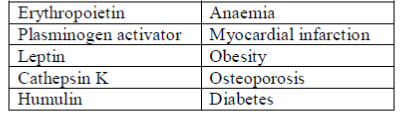
18. Match the therapeutics in Column-I with their applications in Column-II
Column-I Column-II
P. Erythropoietin 1. Diabetes
Q. Plasminogen activater 2. Obesity
R. Leptin 3. Anemia
S. Cathespin K 4. Myocardial
T. Humulin 5. Osteoporosis
6. Cancer therapy
(a) P–3, Q–6, R–2, S–4, T–1
(b) P–5, Q–4, R–6, S–3, T–1
(c) P–3, Q–4, R–2, S–5, T–1
(d) P–5, Q–6, R–4, S–3, T–1
Ans. (c)
Sol. The correct matching for the following are
19. The 2009 Nobel prizes were awarded to worrk on
(a) human papilloma virus and ribosome
(b) Helicobactor pylori and human papilloma
(c) ribosome and telomerase
(d) telomerase and Helicobacoter pylori
Ans. (c)
Sol. The 2009 Nobel prize in chemistry was awarded to studied of the structure and function of ribosome
And in Physiology or medicine, it is awarded to the discovery of how chromosomes are protected by telomeres and the enzyme telomerase.
20. Which of the following statements about yeast are correct?
P. Yeast are fungi
Q. Yeast can form pseudohyphae
R. Yeast reproduce asexually by budding
S. Yeast are facultative anaerobes
T. All yeast are pathogenic
U. All yeast are dimorphic
(a) P, Q, R and S
(b) R, S, T and U
(c) P, R, S and U
(d) Q, R, S and T
Ans. (c)
Sol. Yeast are eukaryotic microorganisms classified as members of the fungs kingdom. The correct statements regarding yeast are
(i) Yeast are fungi
(ii) Yeast reproduce asexually bu budding
(iii) Yeast are facultative anaerobes
(iv) All yeast are dimorphic
21. An L shaped wire PQR carrying a current i is placed at a distance s from the origin (see the figure below). The length PQ is/such the/>> s. The mangnitude of the magnetic field B at origin O is
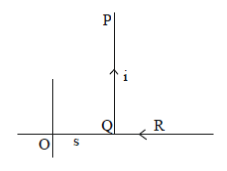
(a) 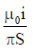
(b) 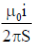
(c) 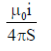
(d) 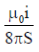
Ans. (c)
Sol. Magnetic field due to QR wire at O
BQR = 0
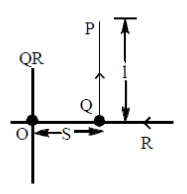
Magnetic field to PQ wire at O
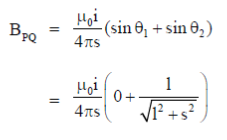
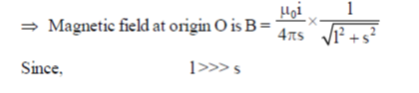

22. The current (in A) in the given circuit, assuming the internal resistance of the batteries to be negligible, is
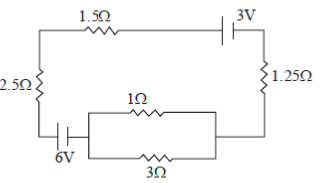
(a) 1/4
(b) 1/2
(c) 3/4
(d) 9/8
Ans. (b)
Sol.
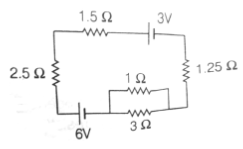
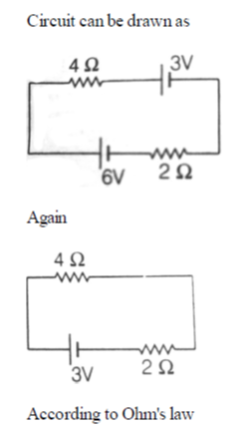

23. A gaseous mass M in the form of a thin disk of radius R is rotating with ω1 as shown in the figure below.
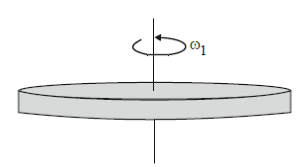
A fraction M/4 of the gas condenses into a thin ring of radius R and the remaining into a concentric disk of radius R/2. The system now rotaes with 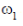 about the same axis. The ratio
about the same axis. The ratio 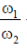 is
is
(a) 16/7
(b) 16/11
(c) 7/8
(d) 4/5
Ans. (d)
Sol. Applying conservation of angular momentum
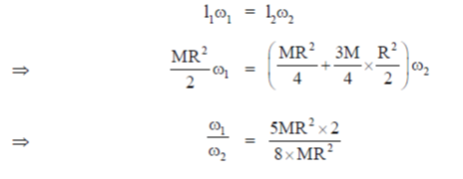

24. A terminal speed (in m/s) of vertically falling raindrop of radius 0.03 cm (g = 9.9 m/s2, 1.8 10–4 poise and Ppower = 1000 kg/m3) is approximately
(a) 0.11
(b) 0.55
(c) 1.10
(d) 2.20
Ans. (c)
Sol. Terminal velocity is given by
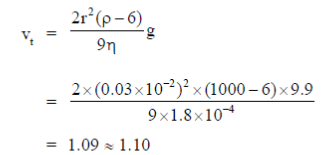
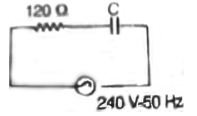
25. In an RC circuit, a resistor of resistance 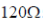 and a capacitor are connected to a 240 V, 50Hz ac source. The circuit takes a current of 1.2A. The reactance of the capacitor
and a capacitor are connected to a 240 V, 50Hz ac source. The circuit takes a current of 1.2A. The reactance of the capacitor 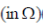 is
is
(a) 80
(b) 120
(c) 160
(d) 240
Ans. (c)
Sol. Current drawn in R-C circuit is given by
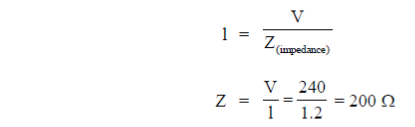
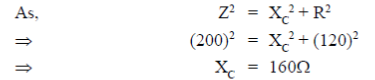
26. A bob of mass m of a long pendulum of length L is at a horizontal position P (see figure below). When released, it hits a ball of mass m placed at a position Q. Assume the collision to be elastic. After hitting the ball at Q, the bob will attain a height (with respect to Q) of
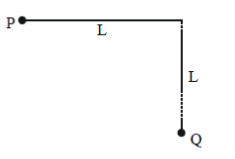
(a) 0
(b) L/4
(c) L/2
(d) L
Ans. (d)
Sol.
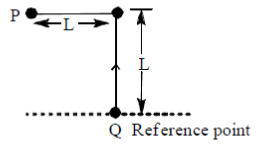
By conservation of energy for bob P
(V = velocity of bob from P to Q)

Since, collision is elastic so, this velocity is completely transferred to bod Q.
Now, applying conservation of energy for bob Q
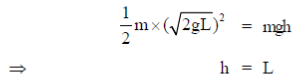
27. Signal recognition particles (SRPs) are
(a) protein-DNA complexes involved in protein sorting
(b) protein-RNA complexes involved in protein sorting
(c) protein-RNA complexes involved in RNA splicing
(d) protein-RNA complexes involved in cell cycle
Ans. (b)
Sol. The signal recognition particle (SRP) is a protein -RNA complex that recognises and transports specific proteins (i.e, protein sorting) to the endoplasmic reticulum in eukaryotes and the plasma membrane in prokaryotes.
28. M Phase promoting factor (MPF) facilitates cells to move from G2 to M phase during the cell cycle. The sudden decline in MPF at the end of the M Phase is due to
(a) degradation of CDKs
(b) degradation of cyclins
(c) the reduced expression of cyclins
(d) an increase in the ratio of cell volume and genome
Ans. (b)
Sol. Maturation promoting factor or M-phase promoting factor in the cyclin-CDK complex that stimulates the mitotic and meiotic phases of the cell cycle. It facilitates cells to move from G2 to M-phase.The sudden decline in MPF at the end of the M-phase is due to degradation of cyclins.
29. Which of the following statements are true regarding the dolichol phosphate pathway?
P. a 14-residue precursor oligosaccharide chain is synthesized in the ER
Q. a 14-residue precursor oligosaccharide chain is synthesized in the Golgi complex
R. it helps in N-linked glycosylation of proteins
S. it helps in O-linked glycosylation of proteins
(a) P and R
(b) Q and R
(c) Q and S
(d) P and S
Ans. (a)
Sol. Statements P and R are true regarding the dolichol phosphate pathway.
The oligosaccharide substrate for the N-linked protein glycosylation is assembled at the membrane of the endoplasmic reticulum. Dolichyl pyrophosphate serves as a carrier in this biosynthetic pathway.
30. Which one of the following is not true about Klenow, fragment?
(a) It is proteolytic cleavager product of DNA polymerase I
(b) It has 5'  3' polymerase activity
3' polymerase activity
(c) It has 3' 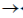 5' exonuclease activity
5' exonuclease activity
(d) It has 5' 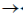 3' exonuclease activity
3' exonuclease activity
Ans. (d)
Sol. The Klenow fragment is a large protein fragment produced when DNA polymerase 1 from E.coli is enzymatically cleaved by the protease subtilisin. First reported in 1970, it retains the 5' 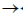 3' polymerase activity and the 3'
3' polymerase activity and the 3' 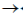 5' exonulcease activity from removal of precoding nucleotides and proofreading, but loses its 5'
5' exonulcease activity from removal of precoding nucleotides and proofreading, but loses its 5' 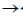 3' exonuclease activity.
3' exonuclease activity.
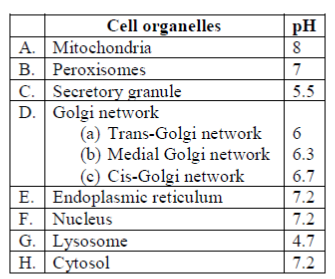
31. In which one of the following options are the cellular compartments arranged in the increasing order of their pH?
(a) Nucleus, mitochondrial matrix, tans-Golgi network, lysosome
(b) Lysosome, nucleus, trans-Golgi network, mitochondrial matrix
(c) Lysosome, trans-Golgi network, nucleus, mitochondrial matrix
(d) Lysosome, nucleus, mitochondrial matrix, trans-Golgi network
Ans. (c)
Sol. The cellular compartments arranged in the increasing order of their pH correctly are
Lysosomes < trans-Golgi network < nucleus < mitochondria
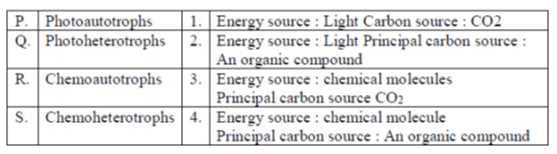
32. Match the entries in Column-I with those in Column-II
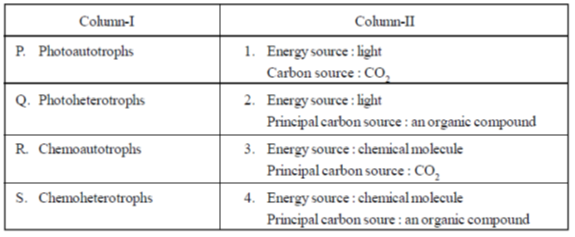
(a) P–1, Q–2, R–3, S–4
(b) P–3, Q–2, R–4, S–1
(c) P–2, Q–4, R–1, S–3
(d) P–4, Q–3, R–2, S–1
Ans. (a)
Sol. The correct matching is
33. Which one of the following is NOT a saturated fatty acid?
(a) Palmitic acid
(b) Stearic acid
(c) Oleic acid
(d) Myristic acid
Ans. (c)
Sol. Olecic acid is an unsaturated fatty acid that is the most widely distributed and abundant fatty acid in nature. A fatty acid is a carboxylic acid with a long aliphatic tail (chain) which is either saturated (single C — C bond) pr unsaturated (has C = C double bonds or triple bonds).
34. The feeding relationship among the species in community determine the community's
(a) secondary succession
(b) ecological niche
(c) Oleic acid
(d) Myristic acid
Ans. (c)
Sol. Trophic structure is a key factor in community dynamics. The trophic structure of a community is determined by the feeding relationship between organisms. This host-parasite and potential competitive interactions. Ecologists depicts the trophic structure of a community in a food chain.
35. Which one of the following technique can be used to find whether a given sample contains glucose or galactose?
(a) Paper chromatography
(b) Thin layer chromatography
(c) NMR spectroscopy
(d) UV spectroscopy
Ans. (c)
Sol. NMR or nuclear magnetic resonance spectroscopy is a research technique that exploits the magnetic properties of certain atomic nuclei.
It determines the physical and chemical properties of atoms or mleucles in which they are contained. Therefore, this technique is used to determine whether the given sample contains glucose or galactose.
36. Match the entries in Column-I with those in Column-II
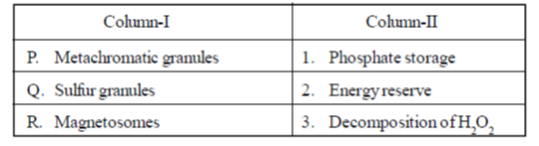
(a) P–1, Q–2, R–3
(b) P–2, Q–3, R–1
(c) P–2, Q–1, R–3
(d) P–3, Q–2, R–1
Ans. (a)
Sol. The correct matching for the following are :
(i) Metachromatic granucles stores phosphate bodies that are present in some species of bacteria.
(ii) Sulfur granules are the storage form of sulfur which are deposited intacellularly. They are common in bacteria that uses hydrogen sulfide as an electron source.
(iii) The magnetosomes, which were identified as membrane bound paticles of a magnetic iron mineral, enable the bacteria to orient themselves and swim along the lines of magnetic field, a phenomenon known as magnetotaxis.
37. Which of the following statements pertaining to 2D gel electrophoresis of proteins is/are CORRECT?
P. while perpairing the sample from a tissue, the sample should be dissolved in SDS
Q. The duration for which SDS gel in run should not vary to ensure reproducibility
(a) Only P
(b) Only Q
(c) P and Q
(d) Neither P nor Q
Ans. (d )
Sol. Neither P or Q is correct because two-dimensional gel electrophoresis (2-D electrophoresis) is a powerful and widely used method for the analysis complex protein mixtures extracted from cells, tissues, or other biological samples. This technique separate proteins in two steps, according to two independent properties : the first-dimensions is isoelectric focusing (IEF), which separates proteins according to their isoelectric points (pl); the second-dimension is SDS-polyacrylamide gel electrophoresis (SDS-PAGE), which separates proteins according to their molecular weights (MW). In this way, complex mixtures consisted to thousands of differents proteins can be resolved and the relative amount of each protein can be determined.
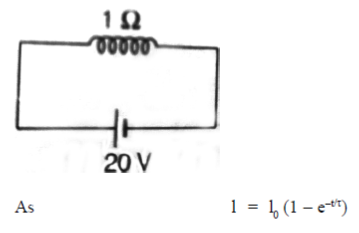
38. An inductive coil of resistance 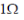 is connected to a 20V battery. Neglect the internal resistance of the battery. The value of the induced emf (in V) in the coil at an instant when the current has risen to one-fourth of its steady value is
is connected to a 20V battery. Neglect the internal resistance of the battery. The value of the induced emf (in V) in the coil at an instant when the current has risen to one-fourth of its steady value is
(a) 5
(b) 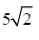
(c) 10
(d) 15
Ans. (d )
Sol.
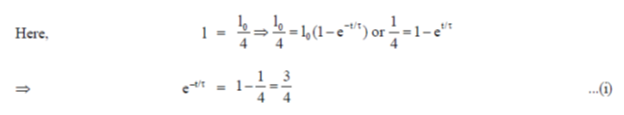
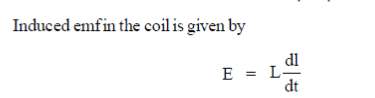
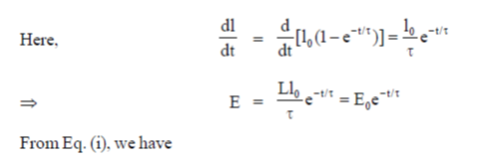
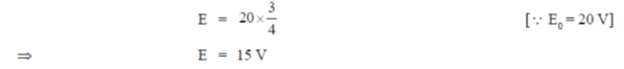
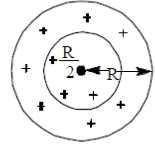
39. A charg +Q is uniformly distributed in a sphere of radius R. The magnitude of the electric field E at a distance 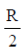 from the centre of the sphere is
from the centre of the sphere is
(a) 0
(b) 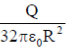
(c) 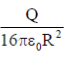
(d) 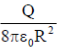
Ans. (d )
Sol. As we know that
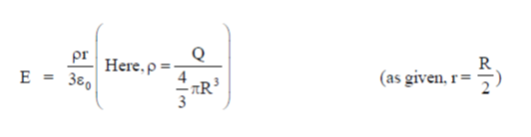
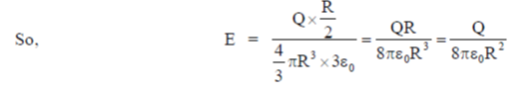
40. A vibrating string of length l has mass m. It vibrates with a fundamental frequency when stretched by a force of 1N. This string will vibrate with second harmonic (i.e., first overtone) if the force is increased to
(a) 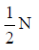
(b) 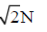
(c) 4N
(d) 8N
Ans. (c)
Sol. As we know that, frequency of vibration in the string is given by v = 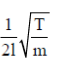
41. Electrons are emitted in photoelectric effect, and beta particles are emitted in radioactive decay of nuclei. Which one of the following statements is CORRECT?
(a) The energy of photoelectrons is much greater than that of beta particles
(b) The energy of beta particles is much greater than that of photoelectrons
(c) The energies of photoelectrons and beta particles are of same order
(d) Beta particles and photoelectrons have different masses
Ans. (b)
Sol. When a photon of light this metal surface, a photoelectron is emitted. Some of the energy of photon is required to free electron from the surface and the remaining energy becomes kinetic energy of electron.
However, beta particles are high energy, high speed electrons or positrons emitted by certain types of radioactive nuclei. Therefore, the energy of beta particles is much more than the electrons emitted in photoelectric effect.
42. Two sources of light are coherent if they emit radiation of
(a) unequal intensities, same wavelength and same phase
(b) equal intensity, same wavelength and different phases
(c) unequal intensities, same wavelength and different phases
(d) equal intensity, different wavelengths and different phases
Ans. (a)
Sol. Two sources of light are said to be coherent if they emit light of same wavelength, same phase differences and unequal intensities.
43. Which one of the following schematics CORRECTLY depicts the variation of conductance as a function of membrane potential for the voltage-gated K+-channel?
(a) 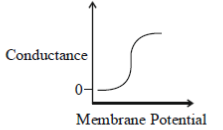
(b) 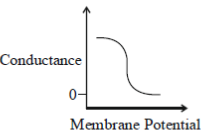
(c) 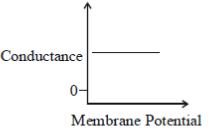
(d) 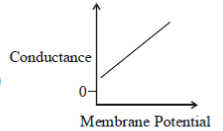
Ans. (a)
Sol.
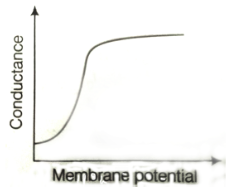
Potassium ions are largely responsible for maintaining the outward current during a prolonged depolarisation of the membrane. The relationship between the potassium current and the membrane potential is linear passing through zero at 10-20mV above the resting potential. This is due to the fact that potassium conductance is a continuous function of time which rises when the nerve is depolarised and falls when the nerve is repolarized. The rate at which the potassium conductance is reduced on repolarisation increases with membrane potential.
44. In a xenogenic cell based therapy, the donor and recipient belong to different species. In which of the following, do the donor and recipient belong to the same species?
P. Autologous
Q. Allogenic
R. Syngeneic
(a) Only P
(b) Only P and Q
(c) Only P and R
(d) P, Q and R
Ans. (d)
Sol. In P, Q and R the donor and recipient belong to the same species. These are explained as
(i) Autologous in blood transfusion and transplantation, is a situation in which the donor and recipient are the same person.
(ii) Allogeneic means genetically different because of being derived from separate individuals of the same species.
(iii) Syngeneic means genetically identical, or sufficiently identical and immunologically compatible as to allow for transplantation.
45. According to the following schematic, which shows the effect of the ligand L on the allosteric protein P,
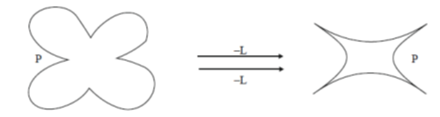
(a) L has to be an allosteric activator
(b) L has to be an allosteric inhibitor
(c) L can be either an allosteric activator or and allosteric inhibitor
(d) L is not an allosteric modulator of the protein
Ans. (a)
Sol.
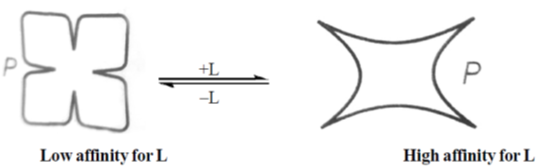
P is an allosteric protein, when ligand L bind with allostric protein, its activity get enhance, i.e. allosteric regulation of protein takes place.
The site to which the effector binds is called as allosteric site, which allows effector to bind to the protein, resulting in conformational change. As effectors enhance the protein's activity thus, reffered to as allosteric activators, whereas those that decrease the protein's activity are called allosteric inhibitor. Therefore, option (a) is correct i.e., L is an allosteic activator.
46. Which one(s) among helicase, primate, telomerae and topoisomerase can form phosphodiester bonds?
(a) Only primase
(b) Primase and telomerase only
(c) Primase, telomerase and topoisomerase only
(d) All the four enzymes
Ans. (c)
Sol. Primase catalyses the synthesis of a short RNA segment (called a primer) complementary to a single stranded DNA template and telomerase is a reverse trnascriptase that carries its own RNA molecule, which is used as a template when it elongates telomeres. Enzyme topoisomerases bind to either single stranded or double stranded DNA and cut the phosphate backbone of the DNA. This intermediate break allows the DNA to be untangled or unwound, and at the end of this process the DNA is joined again.
47. Type II hypersensitivity
(a) in antibody independent
(b) in complement independent
(c) is mediated by CD8+T cells
(d) involves antibody mediated destruction of cells
Ans. (d)
Sol. Type II hypersensitivity is antibody dependent cell mediated cytotoxicity (ADCC). In this the antibodies produced by the immune response bind to antigens on the patient's own cell surfaces and causes destruction of cells.
48. Which of the following statements relating to photosynthesis are CORRECT?
P. Carotenoids protect against toxic oxygen species
Q. When plants utiize blue light, they can harness more energy than when they utilize red light
R. The porphyrin ring in both chlorophyll and bacteriopheophytin has magnesium
S. Chemical modification of the porphyrin ring alters its absorphtion spectrum
T. The Z-scheme, depicting the flow of electrons in phosynthesis, is based on oxidation potentials
U. Carboxysomes are subcellular structures present in certain prokaryotes
V. Efflux of magnesium from the thylakoid lumen into the stroma helps in the activation of RuBisCo
(a) P, Q and R
(b) Q, R, S and V
(c) R, S, T and V
(d) P, S, T, U and V
Ans. (d)
Sol. The only correct statements relating to photosynthesis are
P-Carotenoids protect against toxic oxygen species.
S-Chemical modification of the porphyrin ring alters its absorption spectrum.
T-The Z-scheme depicting the flow of electrons in hoptosynthesis is based on oxidation potentials.
U-Carboxysomes are sucellular structures present in certain prokaryotes.
V-Efflux of magnesium from the thylakoid lumen into the stroma helps in the activation of RuBisCo.
49. Which of the following membranes have a proton-pumping ATPases?
BPM Bacterial plasma membrane
CIM Chloroplast inner membrane
TGM Trans-Golgi membrane
LM Lysosomal membrane
MIM Mitochondrial inner membrane
VM Vacuolar membrane
(a) BPM, CIM, MIM and VM
(b) BPM, TGM, MIM and VM
(c) CIM, LM, MIM and VM
(d) BPM, LM, MIM and VM
Ans. (a)
Sol. The following membrane have a proton pumping ATPase, i.e. BPM-Bacterial plasma membrane
CIM-Chloroplast inner membrane
MIM-Mitochondrial inner membrane
VM-Vacuolar membrane
50. Which one of the following statements is NOT CORRECT?
(a) A mass m is enclosed in a spherical shell. The gravitational force on the mass due to another point mass M lying outside the shell is zero
(b) When an object of mas m is in motion uner a garvitational force, both angular momentum and total mechanical energy are conserved
(c) The acceleration due to garvity decreases with incerasing altitiude
(d) The acceleration due to gravity is dependent on the mass of earth
Ans. (a)
Sol. Since, the gravitational force will act between the mass m enclosed in a spherical shell and mass due to another point mass M lying outside the shell.
51. An electromagetic wave with a magnetic field vector 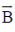 = 100X10–9T cos[(1.8 rad/m)y + (5.4X 106rad/s)t]
= 100X10–9T cos[(1.8 rad/m)y + (5.4X 106rad/s)t]  propagates along
propagates along
(a)  and its electric field vector is along
and its electric field vector is along 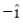
(b) – and its electric field vector is along 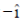
(c) 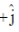 and its electric field vector is along
and its electric field vector is along 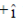
(d) – and its electric field vector is along 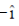
Ans. (b)
Sol. Since, given magnetic field is along Z-zies or k vector and from given option the cross product of propagation of electromagnetic waves and electric field vector should be towards the direction of magnetic field.
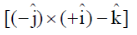
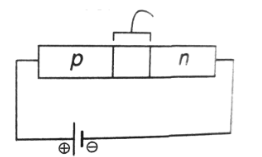
52. Which one of the following statement is NOT CORRECT?
(a) In an unbiased p-n junction, the electric potential of n-side is higher than that of the p-side
(b) When a p-n junction is forward biased, the width of the depletion region increases
(c) When a p-n junction is forward biased, the forward current is due to both electron and hole diffusion
(d) When a p-n junction is forward biased, the potential of the p-side increases
Ans. (b)
Sol. In case of forward biased p-n junction diode
p-terminal is connected with positive terminal and
n-terminal is connected with negative terminal and depletion layer decreases
53. Which one of the following statements is CORRECT for the reaction
2HNO3(aq) + Cu(s) + 2H+(aq) 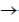 2NO2(g) + Cu2+(g) + 2H2O(l)?
2NO2(g) + Cu2+(g) + 2H2O(l)?
(a) H+ is the oxidizing agent, and Cu is the reducing agent
(b) H+ is the oxidizing agent, and HNO3 is the reducing agent
(c) HNO3 is the oxidizing agent, and Cu is the reducing agent
(d) Cu is the oxidizing agent, and HNO3 is the reducing agent
Ans. (c)
Sol. HNO3 is the oxidising agent and Cu is the reducing agent.
In the reaction
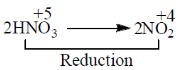
HNO3 itself undergo reduction process and act as an oxidising agent.
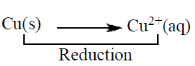
Cu(s) undergoes oxidation process and act as an reducing agent.
54. Which of the following Fishcer projections of glyceraldehyde have identical absolute configuration?
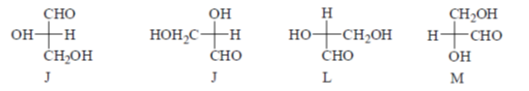
(a) K, L and M
(b) J, K and M
(c) J, K and L
(d) J, L and M
Ans. (c)
Sol. Fischer projection of glyceraldehyde which have identical absolute configuration.
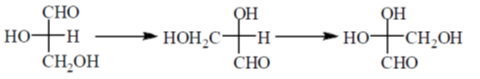
55. In a water solution the concentration of OH– at 25°C is 10–5 mole/liter. The concentration of H3O+ is
(a) 10–19 mole/liter
(b) 10–12 mole/liter
(c) 10–9 mole/liter
(d) 10–2 mole/liter
Ans. (c)
Sol. Conc. of OH– = 10–5 mol L–1
Conc. of H3O+ = ?
pKw = pKa + pKb
or Kw = Ka × Kb
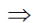 10–14 = H+ × OH–
10–14 = H+ × OH–
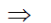 10–14 = H+ × 10–5
10–14 = H+ × 10–5
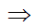 H+ = 10–9 at 25°C
H+ = 10–9 at 25°C
56. The correct orer of basicity of the following amines P, Q, R and S is

(a) S < P < Q < R
(b) R < Q < P < S
(c) Q < P < R < S
(d) P < Q < R < S
Ans. (b)
Sol.
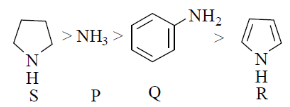
In S structure, the lone pairs are readily available and are exposed thus the hydrogen can easily be extracted by base.
In Q structure, NH2 undergoes resonance
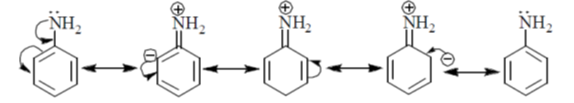
The hydrogen is involved in resonance.
In R structure, the resonance is more pronounced and effective due to the five membered ring structure.
57. The value of x and y in the given Nylon 66 are
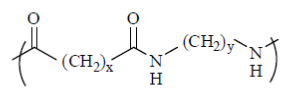
(a) x = 4 and y = 6
(b) x = 6 and y = 4
(c) x = 6 and y = 6
(d) x = 4 and y = 4
Ans. (a)
Sol. Nylon 66 is made of two monomers each containing six carbon atoms, hexamethylene diamine and adipic acid.
Synthesis
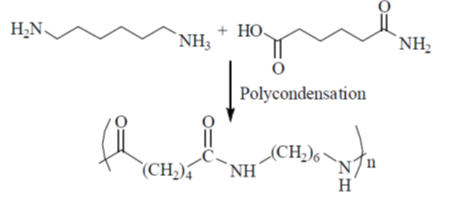
So, here x = 4 and y = 6
58. In the following transformations, the groups R1, R2 and R3 are
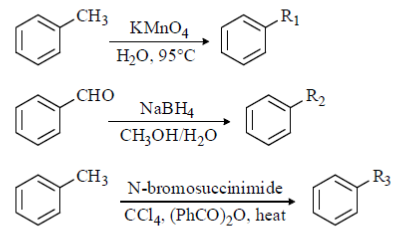
(a) R1 = CH2OH, R2 = CH2Br and R3 = COOH
(b) R1 = COOH, R2 = CH2Br and R3 = CH2OH
(c) R1 = COOH, R2 = CH2OH and R3 = CH2Br
(d) R1 = CH2Br, R2 = COOH and R3 = CH2OH
Ans. (c)
Sol.
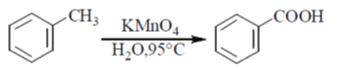
KMnO4 helps the reaction to undergo oxidation
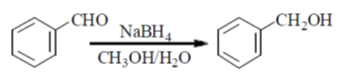
NaBH4 reagent helps in the reduction process
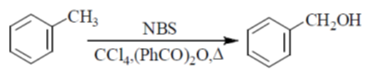
So, R1 = COOH, R2 = CH2OH and R3 = CH2Br
59. The correct match between items of Column-I and Column-II is
Column-I Column-II
P. Fridedel-Crafts reaction 1. Cycloadditon
Q. Baeyer-Viiliger reaction 2. Walden inversion
R. Diels-Alder reaction 3. Oxidation
S. SN2 reaction 4. Aromatic electrophilic substitution
(a) P–3, Q–2, R–1, S–4
(b) P–4, Q–3, R–1, S–2
(c) P–1, Q–2, R–3, S–4
(d) P–4, Q–3, R–2, S–1
Ans. (b)
Sol. Friedel-Craft's reaction is an aromatic electrophillic substitution reaction.
Baeyer-Villiger reaction is an oxidation process.
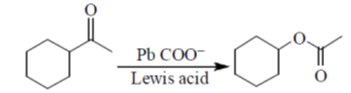
Diels-Alder reaction is a cycloaddition reaction
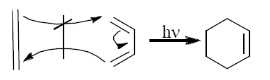
In reaction, the product obtain is walden inversion.
i.e. P-4, Q-3, R-1, S-2
60. The molecular shape of XeF2 is
(a) trigonal bipyramidal
(b) triagonal pyramidal
(c) V-shape
(d) linear
Ans. (d)
Sol. The molecular shape of XeF2 is lenear.
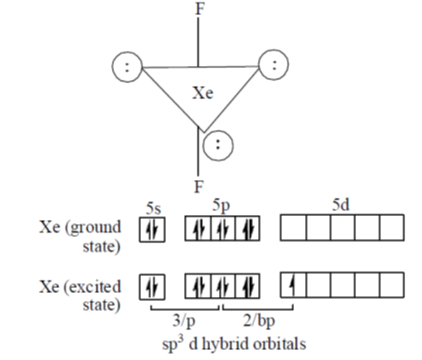
Xe is in sp3 d-hybridised state having three lone pairs of electrons at equatorial position and two bond pairs of electrons on axial position and thus shape is linear with Xe-F bond of 2.0 Å length.
61. The number of signals the 1H NMR spectra of the following molecules P and Q, respectively, are
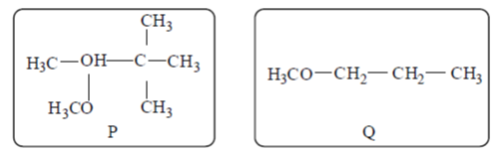
(a) 6 and 4
(b) 4 and 4
(c) 3 and 3
(d) 4 and 3
Ans. (b)
Sol. Sol.
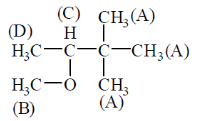
The four signals are observed.
(a) —CH3 (All methyl (A) are equivalent)
Hence, only 1 signal is seen.
(b) —O—Ch3 (1 signal)
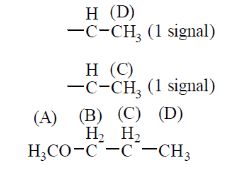
The three signals are observed.
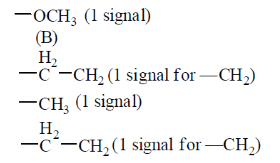
62. Which of the following can be used as a biological weapon?
P. Bacillus anthracis
Q. Bacillus thuringiensis
R. Bacillus subtilis
S. Ebola virus
(a) P and S
(b) P and Q
(c) P and R
(d) Q and S
Ans. (a)
Sol. Baccillus anthracis and Ebola virus can be used as biological weapons.
There are a variety of microorgnaisms that can be used as biological weapons and are chosen bacause they are highly toxic, easily obtainable and inexpensive to produce, easily obtaineable and inexpensive to produce, easily transferable from person to person, can be disperesed in aerosol form or have no known vaccine.
63. The number of acetylated amino sugar(s) in the repeating unit of peptidoglycan is
(a) 1
(b) 2
(c) 3
(d) 4
Ans. (b)
Sol. Peptidoglycan or murein that is unique to bacterial cell wall has a repeating unit consisting of two amino sugars linked via  -1, 4 glycosidic bonds. The resulting linear glycan chains are cross linked at frequent intervals by peptide bridges.
-1, 4 glycosidic bonds. The resulting linear glycan chains are cross linked at frequent intervals by peptide bridges.
64. The genome of an adenovirus is a
(a) linear double stranded DNA
(b) circular double stranded DNA
(c) plus-strand RNA
(d) minus-strand RNA
Ans. (a)
Sol. Adenovirus genomes are linear, non-segmented double stranded (ds)DNA molecules that are typically 26-46 kbp long, containig 23-46 protein coding genes. These viruses causes a wide range of illnesses from mild respiratory infections in young children to life threatening multi-organ disease in pepole with weakened immune system.
65. Which one of the following CANNOT be used to sterilize a heat-labile solution?
(a) Gamma radiation
(b) Ethylene oxide
(c) Autoclaving
(d) UV radiation
Ans. (c)
Sol. A heat-labile solution cannot be sterilised by using autoclave. Heat labile means susceptible to alteration or destruction.
e.g. A heat labile protein can be destroyed at high temperature and autoclaving is done at high temperature upto 121°C.
66. Which one of the following second messengers targets protein kinase A?
(a) cAMP
(b) cGMP
(c) Diacylglycerol
(d) Inositol 1, 4, 5-triphosphate
Ans. (b)
Sol. Protein kinase A is also known as the cyclic AMP-dependent protein kinase or A kinase. It is an enzyme that cocalently decorates protein with phosphate groups. Protein kinase A activity is regulated by fluctating levels or cyclic AMP within the cell.
Thus, protein kinase A is ultimately responsible for essentially all of the cellular responses due to the cyclic AMP second messenger system.
67. Idiogram is
(a) a diagrammatic representation of karyotype of a species
(b) a diagrammatic representation of isotypic antibodies of a species
(c) a diagrammatic representation of the evolutionary tree of various species
(d) an autoradiogram profile of isotypic antipodies of a species obtaned using 125
Ans. (a)
Sol. Ideogram or idiogram is the schematic representation of chromosomes. Also, can be said as a diagrammatic representatino of karyotype of a species. They show the relative size of the chromosomes and their banding patterns.
68. Spindle fibers, formed during the cell division, are composed of
(a) actin
(b) collagen
(c) myosin
(d) tubuin
Ans. (c)
Sol. The spindle fibres, formed during the cell division are made up of microtubules, which are composed of protein-tubulin.
It separate the sister chromatids during cell division, so, that each daughter cell has a complete set of chromosomes.
69. The differential equation representing the family of circles passing through the point (0, –b) and (0, b) is
(a) 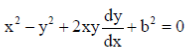
(b) 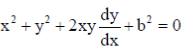
(c) 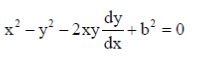
(d) 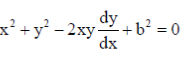
Ans. (b)
Sol. General equation of circle is
x2 + y2 + 2gx + 2fy + c = 0 ...(i)
Circle passes through point (0, b), (0, – b)
 b2 + 2fb + c = 0 ...(ii)
b2 + 2fb + c = 0 ...(ii)
and b2 – 2fb + c = 0 ...(iii)
Subtracting Eq. (iii) from Eq. (ii), we get
4fb = 0
 f = 0
f = 0
From Eq. (ii) b2 + 0 + c = 0
 c = –b2
c = –b2
Putting these values in Eq. (i), we get
x2 + y2 + 2gx + 2fy + c = 0
x2 + y2 + 2gx – b2 = 0 (iv)
On differentiate w.r.t. x, we get
2x + 2yy1 + 2g = 0
 2(x + yy1 + g) = 0
2(x + yy1 + g) = 0
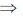 x + yy1 + g = 0
x + yy1 + g = 0
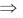 g = –(x + yy1)
g = –(x + yy1)
Putting this value in Eq. (iv), we get
x2 + y2 – 2(x + yy1)x – b2 = 0
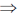 x2 + y2 – 2x2 – 2xyy1 – b2 = 0
x2 + y2 – 2x2 – 2xyy1 – b2 = 0
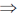 –x2 + y2 – 2xyy1 – b2 = 0
–x2 + y2 – 2xyy1 – b2 = 0
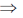 x2 – y2 + 2xyy1 + b2 = 0
x2 – y2 + 2xyy1 + b2 = 0
70. 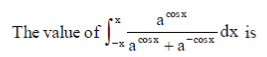
(a) 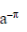
(b) 
(c) 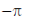
(d) 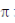
Ans. (d)
Sol. 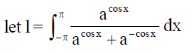
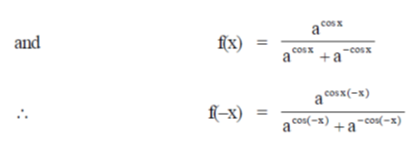

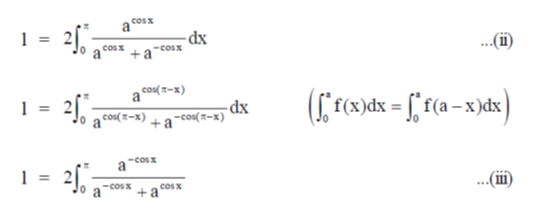
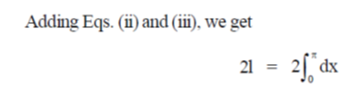
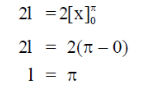
71. The point at which the tangent to the curve x3 + y3 = 6xy is parallel to y-axis (but is not y-axis) is
(a) 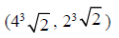
(b) 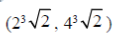
(c) 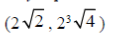
(d) 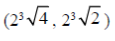
Ans. (d)
Sol. Given curve,
x3 + y3 = 6xy
On differentiate both sides w.r.tx, we get
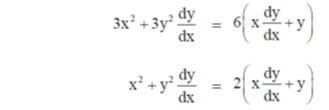
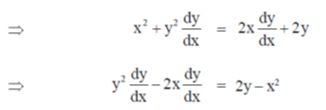
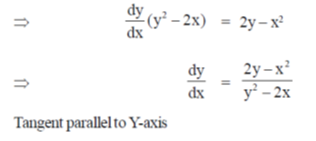
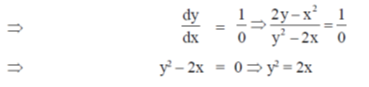

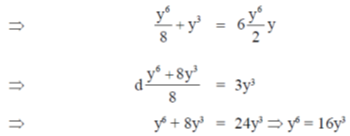
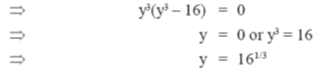
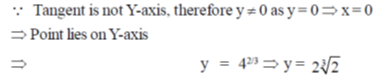
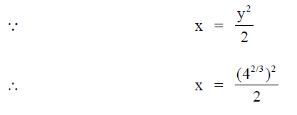
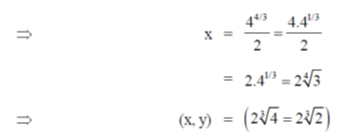
72. For p> 0, q> 0, if the maximum of px + qy, subject to the constraints
0 <x, 0 <y, x + 2y< 10 and 3x + y< 15, exists at points (0, 5) and (4, 3) then the relationship between p and q is
(a) 2p = q
(b) p = 2q
(c) p = 3q
(d) 3p = q
Ans. (a)
Sol. Let objective function, z = px + qy
Maximise at (0, 5) z = p × 0 = q × 5
z = 5q ...(i)
Maximise at (4, 3), z = p × 4 + q × 3
= 4p + 3q ...(ii)
From Eqs. (i) and (ii), we get
4p + 3q = 5q
4p = 2q  2p = q
2p = q
73. The set of complex numbers z, which satisfy the equation |z – 3| + |z + 3| = 10 in the Argand plane, forms
(a) a circle
(b) a parabola
(c) an ellipse
(d) a hyperbola
Ans. (a)
Sol. Equation |z – z1| + |z – z2| = k represents an ellipse, if k > |z1 – z2| = k represents an ellipse, ifk > |z1 – z2|
Here, z1 = 3, z2 = –3
Also |z1 – z2| = 6 < 10
74. If (x + iy)3 = u + iv, then 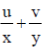 is
is
(a) 4(x2 + y2)
(b) 4(y2 – x2)
(c) 4(x2 – y2)
(d) –4(x2 + y2)
Ans. (c)
Sol. Given, (x + iy)3 = (u + iv)
x3 + (iy)3 + 3 × x × iy(x + iy) = u + iv
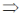 x3 + i3y3 + 3ixy(x + iy) = u + iv
x3 + i3y3 + 3ixy(x + iy) = u + iv
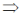 x3 – iy3 + 3x2iy – 3xy2 = u + iv
x3 – iy3 + 3x2iy – 3xy2 = u + iv
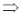 x3 – 3xy2 + i(3x2y – y3) = u + iv
x3 – 3xy2 + i(3x2y – y3) = u + iv
Comparing both sides
u = x3 – 3xy2
v = 3x2y – y3
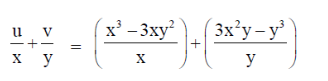
= x2 – 3y2 + 3x2 – y2
= 4x2 – 4y2 = 4(x2 – y2)
75. Saji and Milind are on a treasure hunt. The probability taht Saji will find it is 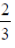 and that both Saji and Milind will find it simultaneously is
and that both Saji and Milind will find it simultaneously is 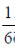 . The probability that Saji alone finds it is
. The probability that Saji alone finds it is
(a) 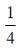
(b) 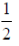
(c) 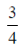
(d) 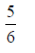
Ans. (b)
Sol. 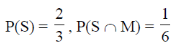
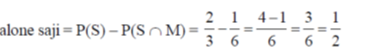
76. Considering the equation 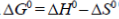 which one of the following statements is NOT CORRECT?
which one of the following statements is NOT CORRECT?
(a) When 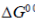 is negative, the reaction is exergonic
is negative, the reaction is exergonic
(b) When 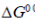 is negative, the reaction can occur spontaneously
is negative, the reaction can occur spontaneously
(c) When 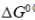 is negative, the molecular disorder decreases during the reaction
is negative, the molecular disorder decreases during the reaction
(d) When 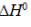 is negative, the reaction is endothermic
is negative, the reaction is endothermic
Ans. (a)
Sol. 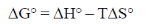
The statement which is not correct is "when 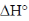 is negative, the reaction is endothermic".
is negative, the reaction is endothermic".
if 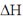 = –ve, than
= –ve, than 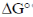 = –ve (spontaneous reaction) for a spontaneous reaction, energy is not required or absorbed by the reaction. So, the reaction cannot be endothermic. It should be exothermic, i.e., energy is released during the reaction.
= –ve (spontaneous reaction) for a spontaneous reaction, energy is not required or absorbed by the reaction. So, the reaction cannot be endothermic. It should be exothermic, i.e., energy is released during the reaction.
77. The difference in the energies of the eclipsed and staggered conformations of ethane at 25°C is approximately
(a) 5.40 kcal/mole
(b) 2.70 kcal/mole
(c) 0.54 kcal/mole
(d) 0.27 kcal/mole
Ans. (c)
Sol. Staggered confomation is always stable than eclipsed conformation as H's are far away in the staggered conformations. So, steric hinderance is not present in staggered. This form is stable by 12.5 kJ/mol, i.e. 2.70 kcal/mol, then eclipsed conformation.
78. Which one of the following statements is NOT CORRECT?
(a) The second ionization potential of an atom is larger than its first ionization potential
(b) Atomic size increases from top to bottom in a group of the periodic table
(c) Electron affinity of an atom is the energy required to add an electron to its oute most orbit
(d) Electronegativity of an atom is its ability to attract electrons towards itself
Ans. (b)
Sol. Electron affinity of an atom is defined as the amount of energy released when a gaseous atom acepts the electron to form gaseous anion.
79. Name of the compound [CO(NH3)6] Cl3 is
(a) cobalt (III) hexammine chloride
(b) hexaamminecobalt chloride (III)
(c) hexaamminecobalt trichloride
(d) hexaamminecobalt (III) chloride
Ans. (c)
Sol. [CO(NH3)6]Cl3
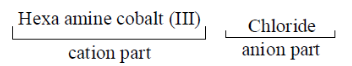
To find the oxidation state of cobalt
[CO(NH3)6]3+
x + 0 = +3
x = +3
Oxidation state of cobalt is + 3.
80. Which one of the following molecules has dipole moment?
(a) PCl3
(b) BCl3
(c) CO2
(d) N2
Ans. (d)
Sol.
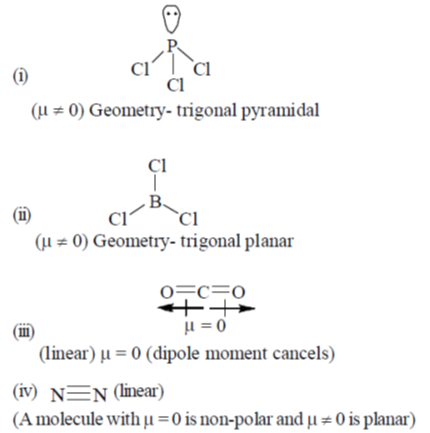
81. In a photochemical reaction, light is involved in
(a) initiation step only
(b) propagation step only
(c) termination step only
(d) propagation and termination steps
Ans. (a)
Sol. In a photochemical reaction, light is involved in initiation step which helps the reaction to proceed by forming free radicals.
82. Proteasomes are
(a) proteomes of lysosomes
(b) protein complexes which recognize and degrade ubiquitinated proteins
(c) protein and cholestreol complexs which help in cholestero transport
(d) protein and RNA complexes which are involved in mRNA splicing
Ans. (b)
Sol. Proteasomes are protein complexes inside all eukaryotes, archaea and in some bacteria. Their main function is to recognise and degrade unneeded or damaged, i.e., ubiqnuitinated proteins by proteolysis, a chemical reaction that breaks peptide bonds.
83. For the function 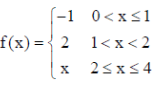
(a) limits exists but is not continuous at 1
(b) limits exists and is continuous at 1
(c) limits exists but is not continuous at 2
(d) limit exists and is continuous at 2
Ans. (d)
Sol. Given,
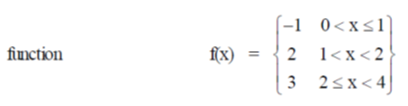
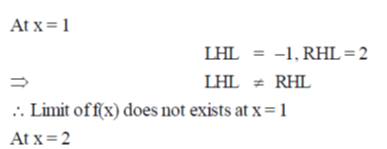
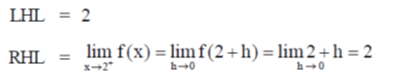
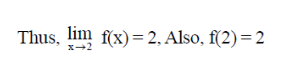
Hence, limit of f(x) exists and f(x) is continuous at
a + x = 2
84. The minimum value of the function x3 – 6x2 + 9x + 10 in the interval [0, 4] is at
(a) 1 only
(b) 1 and 3
(c) 0 and 3
(d) 3 only
Ans. (c)
Sol. Consider, f(0) = 2
f(1) = 14
f(3) = (3)3 – 6(3)2 + 9(3) + 10
= 27 – 6 × 9 + 27 + 10
= 64 – 54 = 10
Minimum value of f(x) is 10 at 0 and 3.
Alternate Method
Let f(x) = x3 – 6x2 + 9x + 10
Differentiable w.r.t.x
f(x) = 3x2 – 12x + 9
For maxima or minima f(x) = 0
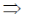 3x2 – 12x + 9 = 0
3x2 – 12x + 9 = 0
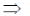 3(x2 – 4x + 3) = 0
3(x2 – 4x + 3) = 0
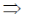 x2 – 4x + 3 = 0
x2 – 4x + 3 = 0
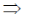 x2 – 3x – x + 3 = 0
x2 – 3x – x + 3 = 0
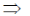 x(x – 3) – 1(x – 3) = 0
x(x – 3) – 1(x – 3) = 0
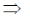 (x – 3) (x – 1) = 0
(x – 3) (x – 1) = 0
 x = 1, 3
x = 1, 3
Now, f(0) = 10
f(1) = 14
f(3) = 10 and f(4) = 14
Hence, f is minimum at x = 0 and 3.
85. The equation of the plane passing through the line of intersection of the planes 3x + 2y = 5 and x + y + 2z + 1 = 0, and containing the point (1, 2. 3) is
(a) 14x – 9y + 2z = 2
(b) 14x – 9y – 2z = 26
(c) 9x – 14y – 2z = 31
(d) 9x + 14y + 2z = 43
Ans. (b)
Sol. Equation of line of intersection of planes is
(3x + 2y – 5) + 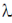 λ(x + y + 2z + 1) = 0
λ(x + y + 2z + 1) = 0
 it contains the point (1, 2, 3)
it contains the point (1, 2, 3)
(3 × 1 + 2 × 2 – 5) + 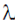 λ(1 + 2 + 2 × 3 + 1) = 0
λ(1 + 2 + 2 × 3 + 1) = 0
(3 + 4 – 5) +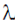 λ(1 + 2 + 6 + 1) = 0
λ(1 + 2 + 6 + 1) = 0
2 + 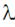 λ(10) = 0
λ(10) = 0
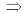 2 = –10λ
2 = –10λ
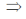
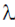 =
= 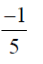
Required equation
(3x + 2y – 5) + 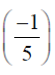 (x + y + 2z + 1) = 0
(x + y + 2z + 1) = 0
15x + 10y – 25 – x – y – 2z – 1 = 0
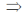 14x + 9y – 2z = 26
14x + 9y – 2z = 26
86. If 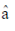 and
and  are unit vectors inclined at an angle, then
are unit vectors inclined at an angle, then 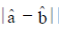 is
is
(a) 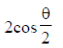
(b) 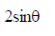
(c) 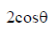
(d) 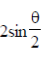
Ans. (d)
Sol. a – b|2 = (a – b)(a – b) = |a|2 + |b|2 – 2ab
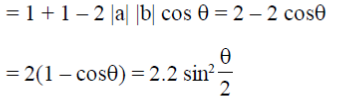
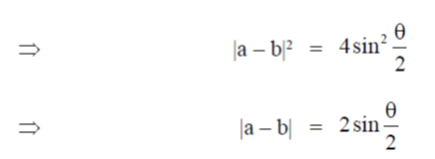
87. For A = 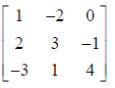 the value of the determinant of adjoint of A is
the value of the determinant of adjoint of A is
(a) 576
(b) 529
(c) 441
(d) 361
Ans. (b)
Sol. 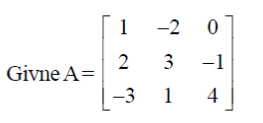
|A| = 1(12 + 1) +2(8 – 3) + 0
= 13 + 10 = 23
Clearly, |adj A| = |A|3 – 1 = |A|2 = (23)2 = 529
88. The converse of the statement "x = 2 implies x2 = 4" is
(a) x2 = 4 implies x = 2
(b) x2 = 4 implies x = –2
(c) x2 = 4 implies (x = 2 or x = –2)
(d) x2 = 4 implies (x = 2 or x = –2)
Ans. (c)
Sol. |A| = 1(12 + 1) +2(8 – 3) + 0
= 13 + 10 = 23
Clearly, |adj A| = |A|3 – 1 = |A|2 = (23)2 = 529
89. The number of functions from the set {a, b, c, d} to set {1, 2, 3} is
(a) 12
(b) 36
(c) 64
(d) 81
Ans. (d)
Sol. Consider, x2 = 4  x2 – 4 = 0
x2 – 4 = 0
 (x – 2)(x + 2) = 0
(x – 2)(x + 2) = 0
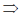 x = 2 or x = –2
x = 2 or x = –2
Hence, the converse of given statement is x2 = 4 implies (x = 2 or x = – 2)
90. 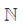 is the set of natural number,
is the set of natural number, 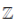 is the set of integers,
is the set of integers, 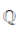 is the set of rational numbers,
is the set of rational numbers, 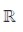 is the set of real numbers and
is the set of real numbers and 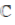 is the set of complex numbers. There is bijection between
is the set of complex numbers. There is bijection between
(a) 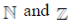
(b) 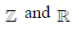
(c) 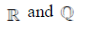
(d) None of these
Ans. (d)
Sol. None of these
91. The removal of bursa of Fabricius from a chicken results in
(a) a delayed rejection of skin graft
(b) low serum levels of antibodies
(c) anemia
(d) a marked decerases in the number of circulating T lymphocytes
Ans. (b)
Sol. The removal of the bursa of fabricius in newly hatched chicks severely impairs the ability of the adults birds to produce antibodies, i.e. low serum of antibodies.
As in birds, the bursa of fabricius is the site of hematopoiesis, it is a specialised organ that is necessary for B cell development in birds.
92. A mouse, which lacks thymus, is called
(a) SCID mouse
(b) NUDE mouse
(c) BEIGE mouse
(d) CBA/N mouse
Ans. (b)
Sol. A nude mouse is a laboratory mouse form a strain with a genetic mutation that causes a deteriorated or absent thymus resulting in an inhibited immune system.
93. Which one of the following is a gratuitous inducer of the Iac operon?
(a) Galactose-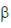 (1, 6)-glucose
(1, 6)-glucose
(b) Galactose-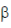 (1, 4)-glucose
(1, 4)-glucose
(c) O-Nitrophenylgalactoside
(d) Isopropyl-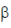 -thiogalactoside
-thiogalactoside
Ans. (d)
Sol. IPGT (Isoprophyl- 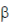 -thiogalactoside) is called a gratuitous inducer of lac operon because it binds to the repressor protein and protects it from binding to the operon, thus, resulting in continuous production of the gene products from thelac operon.
-thiogalactoside) is called a gratuitous inducer of lac operon because it binds to the repressor protein and protects it from binding to the operon, thus, resulting in continuous production of the gene products from thelac operon.
94. Which of the following statements pertaining to cell and tissue culture are CORRECT?
P. In a tissue culture incubator, increasing the partial pressure of CO2 results in a decerase of the pH of the medium
Q. An inactive telomerase is required for a cell to achieve immortality
R. Antibiotics are added to the culture media to prevent microbial contaimination
S. Hayflick limit refers to the number of cells that can grow in a culture flask
T. Serum proteins are required for the adhesion of cells to the surface of a solid substrate
(a) P, Q, S and T
(b) P, Q and T
(c) P, R and T
(d) Q, R and T
Ans. (a)
Sol. The only correct statements pertaining to cell and tissue culture are
P-In tissue culture incubator, increasing the partial pressure of CO2 result in a decrease of tghe pH of the medium
Q-An active telomerase is required for a cell to achieve immortality.
S-Hayflick limit refers to the number of cells that can grow in a culture flask.
T-Serum proteins are required for the adhesion of cells of the surface of a solid substrate.
95. In the schematic shown below, P, Q, R, S, T, U and V are metabolites.
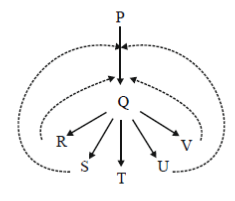
The dotted lines denote
(a) sequential feedback inhibition
(b) negative feedback inhibition
(c) repression
(d) cumulative feedback inhibition
Ans. (d)
Sol. In the given figure P, Q, R, S, T, U and V metabolities shows cumulative feedback inhibition.
In some inhibition processes at high concentration each compound causes only partial inhibition but when mixtures of the inhibitors are present at nearly saturating concentration their effect is cumulative.
Thus, here we come to a conclusion that each inhibitor/metabolite is able to cause only partial inhibition, collectively they can cause almost complete inhibition of the enzyme.
96. The correct match between items of Column I and Column II is
Column I Column II
P. Co2+ (aq) 1. Colorless
Q. Zn2+ (aq) 2. Blue
R. Cu2+ (aq) 3. Pink
S. Ni2+ (aq) 4. Green
(a) P–3, Q–1, R–2, S–4
(b) P–4, Q–2, R–1, S–3
(c) P–3, Q–4, R–2, S–1
(d) P–2, Q–4, R–1, S–3
Ans. (a)
Sol. CO2+ (aq)  Pink
Pink
Zn2+ (aq)  Colourless
Colourless
Cu2+ (aq)  Blue
Blue
Ni2+ (aq)  Green
Green
97. Considering the acidities of the given molecules, which one of the following orders is NOT CORRECT?
(a) CH4 < NH3 < H2O < HF
(b) SiH4 < PH3 < H2S < HCl
(c) HF < HCl < HBr < HI
(d) H4O < H3S < H2Te < H2Se
Ans. (d)
Sol. As we move from top to bottom in a group acidity increases. It is evident from the dissociation constants as
H2O < H2S < H2Se < H2Te
Dissociation constant 1 × 10–14 1 × 10–7 1.7 × 10–4 2.3 × 10–3
98. Which one of the following alcohole undergoes dehydration with rearrangemetn involving a methyl migration?
(a) 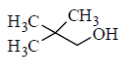
(b) 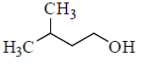
(c) 
(d) 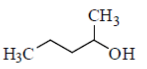
Ans. (a)
Sol.
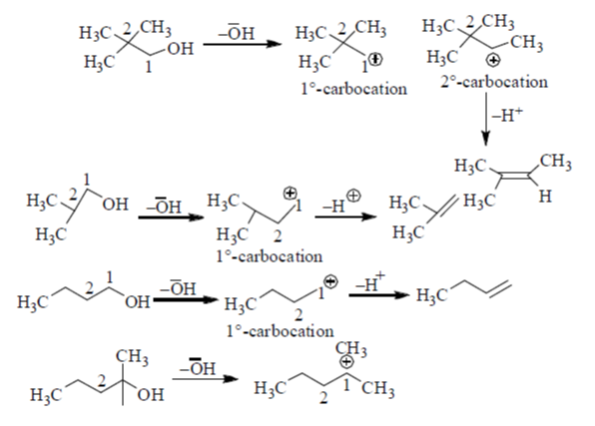
Since, 2°-carbocation is more stable. Hence, methyl group migrates from adjacent position of 1°-carbocation, i.e. from position 2. In compounds mentioned in option (b), (c) and (d), there are no methyl groups present at position 2. Hence these do not involve methyl migration for dehydration.
99. Which one of the following compounds gives acetone as one of the products when treated sequentially with (i) O3 and (ii) Me2S?
(a) 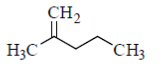
(b) 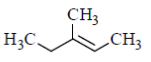
(c) 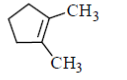
(d) 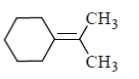
Ans. (d)
Sol. In presence of O3 and Me2S, double bond breaks and convert into corresponding aldehyde or ketones as
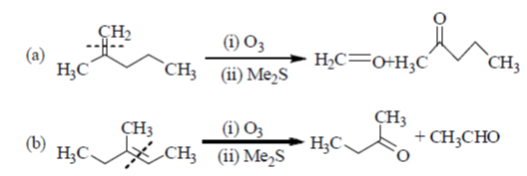
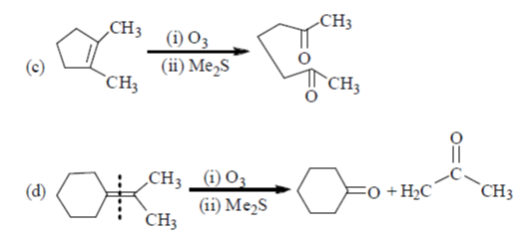
Hence, option 'd' is correct.
100. The units of rate constant (k) of a reaction are 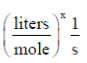 . If the order of the reaction is
. If the order of the reaction is 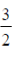 , the value of x is
, the value of x is
(a) 0
(b) 1/2
(c) 1
(d) 3/2
Ans. (b)
Sol. Given, k = 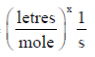
In general for a 'n' order reaction unot of rater constant
(k) = (mole)1–n (litres)n–1 time–1