GATE Chemistry 2018
Previous Year Question Paper with Solution.
1. For low partial pressure of ozone  , the adsorption of ozone on graphite surface is the fully dissociative in nature and follows Langmuir isotherm. Under these conditions, if the dependence of the surface coverage of graphite
, the adsorption of ozone on graphite surface is the fully dissociative in nature and follows Langmuir isotherm. Under these conditions, if the dependence of the surface coverage of graphite 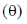 on partial pressure of ozone
on partial pressure of ozone  is given by
is given by  , the value of x is ____________ (Upto two decimal places)
, the value of x is ____________ (Upto two decimal places)
Ans. (0.33)
Sol. 
2. According to Eyring state theory for a bimolecular reaction, the activated complex has
(a) no vibrational degrees of freedom
(b) vibrational degrees of freedom but they never participate in product formation
(c) one high frequency vibration that leads to product formation
(d) one low frequency vibration that leads to product formation
Ans. (d)
Sol. Ewb = hv = kbT hv = kBT, v = 
The frequency of such vibration will be low and average energy will be of the order of kBT.
Correct option is (d)
3. The major product formed in the following reaction is

(a) 
(b) 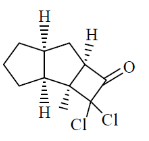
(c) 
(d) 
Ans. (c)
Sol. 
4. The major product of the following intramolecular cycloaddition reaction is

(a) 
(b) 
(c) 
(d) 
Ans. (d)
Sol. 
5. The coordination geometries around the copper ion of plastocyanin (a blue-copper protein) in oxidized and reduced form, respectively are
(a) tetrahedral and square-planar
(b) square-planar and tetrahedral
(c) distorted tetrahedral for both
(d) ideal tetrahedral for both
Ans. (c)
Sol. Both oxidised and reduced form of plastocyanin have distorted Td geometry.
6. The major product formed in the following reactions is

(a) 
(b) 
(c) 
(d) 
Ans. (d)
Sol. Hantzsch Dihydropyridine synthesis





7. The spherical harmonic function  with appropriate values of
with appropriate values of  and m, is an eigenfunction of
and m, is an eigenfunction of  operator. The corresponding eigenvalue is
operator. The corresponding eigenvalue is
(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol. 



8. The temperature derivative of electrochemical potential E at constant pressure,  is given by
is given by
(a) 
(b) 
(c) 
(d) 
Ans. (b)a
Sol. 
9. The water exchange rates for the complex ions follow the order
(a) 
(b) 
(c) 
(d) 
Ans. (d)
Sol. Rate of water exchange 
It also depend upon electronic configuration (for ion having same o.s.) ions having d3 configuration show alter exchange lesser as compared so d7 configuration. Hence order of H2O exchange is

Correct option is (d)
10. For an ionic micelle-forming surfactant near its critical micelle concentration (CMC), the dependence of molar conductivity and surface tension on surfactant concentration is best represented by
(a) 
(b) 
(c) 
(d) 
Ans. (b)
Sol. Ionic micelle forming surfactant near its critical miscelle concentration (CMC)

Correct option is (b)
11. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. (b)
Sol.

12. Two moles of an ideal gas X and two moles of an ideal gas Y, initially at the same temperature and pressure, are mixed under isothermal-isobaric condition. The entropy change on mixing is ______________JK–1.
(Upto one decimal place, Use R = 8.31 JK–1 mol–1)
Ans. (23.05 JK–1)
Sol. 
. 
Correct answer is (23.05 JK–1)
13. Consider the operators , where
, where  and
and  are the position and linear momentum operators, respectively. The commutator,
are the position and linear momentum operators, respectively. The commutator,  is equal to
is equal to
(a) 
(b) 
(c) 
(d) 
Ans. (c)
Sol. 



14. In the 1H NMR spectrum of an organic compound recorded on a 300 MHz instrument, a proton resonates as a quartet at 4.20 ppm. The individual signals of quartet appear at  , 4.19, 4.21 and 4.23 ppm. The coupling constant J in Hz is ___________
, 4.19, 4.21 and 4.23 ppm. The coupling constant J in Hz is ___________
Ans. (6)
Sol. J = 4.19 – 4.17 = 0.02 ppm (since, 1 ppm = 300 Hz)
0.02 ppm = 0.02 × ppm = 6Hz
Correct answer is (6)
15. The bond angle (Ti–C–C) in the crystal structure of

is severely distorted due to
(a) hydrogen-bonding interaction
(b) agostic interaction
(c) steric bulk of the phosphine ligand
(d) higher formal charge on metal.
Ans. (b)
Sol. Distortion in Ti–C–C bond angles is due to agostic interaction
Correct option is (b)
16. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol.

17. The lowest energy transition of the complexes follow the order
transition of the complexes follow the order
(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol. Order of energy for d-d transition is

it depends upon ligand field strength.
Order of ligand field strength
H2O < NH3 < CN–
Correct option is (a)
18. The major product of the following reaction is

(a) 
(b) 
(c) 
(d) 
Ans. (b)
Sol. 
19. The total number of valence electrons in W  is ____________ (Atomic number of W = 74)
is ____________ (Atomic number of W = 74)
Ans. (18)
Sol. w( – Cp) (
– Cp) ( – Cp) (CO)2
– Cp) (CO)2
Electron from w = 6
Electron from  – Cp = 3
– Cp = 3
Electron from  – Cp = 5
– Cp = 5
Electron from 2 CO = 
Hence, number of valence electron is eqal to 18.
20. The energy of a hydrogen molecule in its ground state equilbrium configuration is –31.7eV. Its dissociation energy is ______________eV. (Upto one decimal place)
Ans. (4.5)
Sol.

21. The molar heat capacity of a substance is represented in the temperature range 298K to 400K by the empirical relation  , where b is a constant. The molar enthalpy change when the substance is heated from 300K to 350K is 2 kJ mol–1. The value of b is _____________ J K–2 mol–1. (Upto two decimal places)
, where b is a constant. The molar enthalpy change when the substance is heated from 300K to 350K is 2 kJ mol–1. The value of b is _____________ J K–2 mol–1. (Upto two decimal places)
Ans.
Sol. T1 = 298 K; T2 = 400 K
Cp, m = (14 + bT) JK–1 mol–1



22. In the electron ionization (EI) mass spectra, methly hexanoate, methyl heptanoated and methyl octanoate give the same base peak. The m/z value of the base peak is _____________
Ans. (74)
Sol. 

23. For the radioactive isotope 131I, the time required for 50% disintegration is 8 days. The time required for the 99.9% disintegration of 5.5 of 131I is _____________ days. (Upto one decimal place)
Ans. (80.33)
Sol. 

24. The symmetry label of valence p orbitals of a metal ion in an octahedral ligand field is
(a) tlg
(b) ttu
(c) eg + alg
(d) t2g
Ans. (b)
Sol. The symmetry of label of valence p orbital of a metal ion in an octahedral ligand field is t1u.
25. Based on Wade's rule, the structure-type of [B5H8]– is
(a) closo
(b) nido
(c) arachno
(d) hypho
Ans. (b)
Sol. [B5H8]– = [B5H5]4– = Nido
Correct option is (b)
26. Spectroscopic ground state term symbols of cobalt ions in  respectively are
respectively are
(a) 
(b) 
(c) 
(d) 
Ans. (b)
Sol. [Co(H2O)6]2+, high spin


27. The reaction of equimolar quantities of Fe (CO)5 and OH– gives a complex species X which on further reaction with MnO2 give species Y. X and Y, respectively, are
(a) 
(b) 
(c) 
(d) 
Ans. (d)
Sol. 
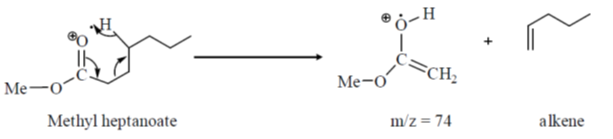
28. The enantiomeric pair, among the following is
(a) 
(b) 
(c) 
(d)  and
and 
Ans.
Sol.


 Both are enantiomers.
Both are enantiomers.
Hence the correct option is (d)
29. In a molecule XY, let  and
and  denote normalized atomic orbitals of atoms X and Y, respectively. A normalized molecular orbital of XY is given by
denote normalized atomic orbitals of atoms X and Y, respectively. A normalized molecular orbital of XY is given by  . The value of the overlap integral of
. The value of the overlap integral of  and
and 
 is ___________ (Upto two decimal place)
is ___________ (Upto two decimal place)
Ans. (0.59)
Sol. For molecule XY if  and
and  are normalized atomic orbital for atoms X and Y
are normalized atomic orbital for atoms X and Y

 where, SXY = overalp integral
where, SXY = overalp integral
(0.56)2 + (0.56)2 + 2 × 0.56 × 0.56 × SXY = 1 {SXY = 0.59}
Correct answer is (0.59)
30. The absorption maxima of two dyes X and Y are 520 and 460 nm, respectively. The absorbance data of these measured in a 1cm path length cell are given in the table below.

The concentration of Y in the mixture is ___________ mM. (Upto two decimal places)
Ans. (7.7967 mM)
Sol. 




Correct answer is (7.7967 mM)
31. The major product in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol.

32. the elimination product of the following reaction is

(a) I2
(b) CH3I
(c) CH3COI
(d) I3
Ans. (c)
Sol. 
33. Number of carbonyl groups present in the final product of the following reaction sequence is

Ans. (4)
Sol. 

Correct option is (4)
34. For the following reaction sequence,

X and Y, respectively, are
(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol.

35. he major product of the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. (d)
Sol. 
Correct option is (d)
36. For a diatomic vibrating rotor, in vibrational level v = 3 and rotational level J, the sum of the rotational and vibrational energies is 11493.6 cm–1. Its equilibrium oscillation frequency is 2998.3 cm–1, anharmonicity constant is 0.0124 and rotational constant under rigid rotor approximation is 9.716 cm–1. The value of J is ___________. (Up to nearest integer)
Ans. (12)
Sol. 

1454.99 = 9.716 J (J +1)
J (J + 1) = 149.7

Correct option is (12)
37. At temperature T, the canonical partition function of a harmonic oscillator with fundamental frequency (v) is given by

For  , the probability of finding the harmonic oscillation in its ground vibrational state is ___________ (Upto two decimal places)
, the probability of finding the harmonic oscillation in its ground vibrational state is ___________ (Upto two decimal places)
Ans. (**)
Sol. Probability of finding particle in ground state




p0 = 0.95
Note: Here partition function give is vibrational partition function with considering zero point energy.

38. A one-dimensional anharmonic oscillator is treated by perturbation theory. The harmonic oscillator is used as the unperturbed system and the perturbation is  (
( is a constant). Using only the first order correction, the total ground state energy of the anharmonic oscillator is
is a constant). Using only the first order correction, the total ground state energy of the anharmonic oscillator is
Note: For aone-dimensional harmonic oscillator 
(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol. 



39. The rate constant of a first order reaction,  is 1.6×10–1 s–1 at 300K. Given that the activation energy of the reaction is 28 kJ mol–1 and assuming Arrhenium behaviur for the temperature dependence, the total time required to obtain 90% of Y at 350K is ___________s. (Upto to one decimal place, use R = 8.31 JK–1 mol–1).
is 1.6×10–1 s–1 at 300K. Given that the activation energy of the reaction is 28 kJ mol–1 and assuming Arrhenium behaviur for the temperature dependence, the total time required to obtain 90% of Y at 350K is ___________s. (Upto to one decimal place, use R = 8.31 JK–1 mol–1).
Ans. (289.6 sec)
Sol. 

40. The strongest band observed in the IR spectrum of the final product of the following reaction appears, approximately at __________×100 cm–1 (Upto one decimal place)
. 
Ans. (17.0)
Sol. 
Correct answer is (17.0)
41. The reaction of PCl3 with PhLi in 1:3 molar ratio yields X as one of the products, which on further treatment with CH3I gives Y. The reaction of Y with n-BuLi gives product Z. The product X, Y and Z respectively, are
(a) 
(b) 
(c) 
(d) 
Ans. (c)
Sol.

Correct option is (c)
42. The  electrons in benzene can be modelled as particles in a ring that follow Pauli's exclusion principle. Given that the radius of benzene is 1.4Å, the longest wavelength of light that is absorbed during an electronic transition in benzene is __________________________________nm. (Upto one decimal place. Use h = 6.6 × 10–34Js, c = 3.0 ×108 ms–1)
electrons in benzene can be modelled as particles in a ring that follow Pauli's exclusion principle. Given that the radius of benzene is 1.4Å, the longest wavelength of light that is absorbed during an electronic transition in benzene is __________________________________nm. (Upto one decimal place. Use h = 6.6 × 10–34Js, c = 3.0 ×108 ms–1)
Ans. (212.1 nm)
Sol. The longest wavelength absorption in the benzene spectrum according to model




43. Second-order are constant for the reaction between  and H2O; n = 2 for X = Cl–)
and H2O; n = 2 for X = Cl–)  and at room temperature varies with the X as
and at room temperature varies with the X as
(a) 
(b) 
(c) 
(d) 
Ans. (b)
Sol. Cl– > H2O > NH3
Correct option is (b)
44. The Latimer diagram of oxygen is given below. The value of x is ___________ V. (Upto two decimal places)

Ans. (1.23)
Sol.



Correct answer is (1.23)
45. The major product formed in the following retro-aldol reaction is

(a) 
(b) 
(c) 
(d) 
Ans. (b)
Sol.

46. The enthalpy of vaporization of a liquid at its boiling point (Tb = 200 K) is 15.3 kJ mol–1. If the molar volumes of the liquid and the vapour at 200K are 100 and 12000 cm3 mol–1, respectively, then the slope  of the liquid-boundary is ___________kPa (Upto two decimal places. Note : 1 Pa = 1 J m–1)
of the liquid-boundary is ___________kPa (Upto two decimal places. Note : 1 Pa = 1 J m–1)
Ans. (6.4339 kPaK–1)
Sol.



= 0.0064339 × 106 = 6433.9 PaK–1 = 6.4339 kPaK–1
47. The O2 coordinated to metal ion centres in oxy-myoglobin and oxy-hemocyanin exists, respectively, as
(a) superoxide and peroxide
(b) superoxide and superoxide
(c) peroxide and peroxide
(d) superoxide and oxygen
Ans. (a)
Sol. O2 coordinated to oxyhemoglobin exist as  while in oxyhemocyanin as
while in oxyhemocyanin as 
Correct option is (a)
48. For an inverse spinel, AB2O4, the A and B, respectively, can be
(a) Ni(II) and Ga(III)
(b) Zn(II) and Fe(III)
(c) Fe(II) and Cr(III)
(d) Mn(II) and Mn(III)
Ans. (a)
Sol. A spinel AB2O4, is inverse spinel if CFSE of A2+ ion in octahedral site is more than CFSE of B3+ ion in oh site.
CFSE of Ni2+ in oh site is more than CFSE of Ga(III).
Correct option is (a)
49. he molar conductivity of a 0.01 M weak acid (HX) at 298K, measured in a conductivity cell with cell constant of 0.4cm–1, is 64.4 Scm2 mol–1. The limiting molar conductivities at infinite dilution of H+ and X– at 298K are 350 and 410 S cm2 mol–1. Ignoring activity coefficients, the pKa of HX at 298K is ___________ (Upto two decimal places)
Ans. (4.105)
Sol. 



50. The spacing between the two adjacent lines of the microwave spectrum of H35Cl , is 6.35 × 1011 Hz, given that bond length D35Cl is 5% greater than that of H35Cl the corresponding spacing for D35Cl is ___________ × 1011 Hz. (Upto two decimal places)
Ans. (**)
Sol. 
2BH – Cl = 6.35 × 1011 Hz
BH – Cl = 3.175 × 1011 Hz




= 2.95 × 1011 Hz
51. Generally, the coordination number and the nature of the electronic absorption band  of lanthanide (III) ion in their complexes are respectively
of lanthanide (III) ion in their complexes are respectively
(a) greater than 6 and sharp
(b) 6 and broad
(c) less than 6 and sharp
(d) greater than 6 and broad
Ans. (a)
Sol. Due to larger size of lanthanoid, they have coordination number generally greater than 6. Also due to deep buried nature of f-orbital they show sharp peak in their absorption spectrum.
Correct option is (a)
52. A tetrapeptide, made up of natural amino acids, has alanine as the N-terminal residue which is coupled to a chiral amino acid. Upon complete hydrolysis, the tetrapeptide gives glycine, alanine, phenylalanine and leucine. The number of possible sequences of the tetrapeptide is ___________
Ans. (4)
Sol. Ala-Phe-Gly-Leu
Ala-Leu-Gly-Phe
Ala-Phe-Leu-Gly
Ala-Leu-Phe-Gly
Correct answer is (4)
53. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. (d)
Sol.

54. The major product formed in the flowing reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol.


Correct option is (a)
55. In the following reaction,

(a) X is the major product and Y is the minor product
(b) X is the only product
(c) Y is the only product
(d) X is the minor product and Y is the major product
Ans. (a)
Sol. Cram's Product :

Correct option is (a)
