GATE Chemistry 2014
Previous Year Question Paper with Solution.
Q.1 – Q.25 : Carry ONE mark each.
1. The maximum non-PV work that a system can perform at constant P is
(a) 
(b) 
(c) 
(d) 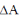
Ans. (b)
Sol. Maximum non-PV work is equal to 
Correct option is (b)
2. Consider the reaction:

The unit of the thermodynamic equilibrium constant for the reaction is
(a) mol L–1
(b) L mol–1
(c) mol2 L–2
(d) dimensionless
Ans. (d)
Sol. 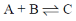
Thermodynamic equation constant is dimensionless.
If equilibrium constant is kp

Correct answer is (d)
3. The number of IR active vibrational normal modes of CO2 is _________
Ans. (3)
Sol. Number of IR active vibrational normal mode of CO2

4. The number of C2 axis in CCl4 is __________
Ans. (3)
Sol. N umber of C2 axis in CCl4 is 3
umber of C2 axis in CCl4 is 3
5. The value of the magnetic quantum number of a px orbital is
(a) –1
(b) 0
(c) +1
(d) undefined
Ans. (4)
Sol. The value of magnetic quantum number of px orbitial is undefined first of all fix the direction of applied magnetic field. Then decide the value of magnetic quantum number.
Correct option is (d)
6. The molecular partition function for a system in which the energy levels are equispaced by 
(a) 
(b) 
(c) 
(d) 
Ans. (d)
Sol. 
Since, energy level are equi-spaced,
if we consider ground state 

This is an exponential series, which is equal to

7. A monoatomic gas, X, adsorbed on a surface, Langmuir adsorption isotherm. A plot of the fraction of surface coverage,  against the concentration of the gas [X], for very low concentration of the gas, is described by the equation
against the concentration of the gas [X], for very low concentration of the gas, is described by the equation
(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol. 


8. At a given temperature and pressure, the ratio of the average speed of hydrogen gas to that of helium gas is approximately _________.
Ans. (1.4 to 1.5)
Sol. 

Correct answer is 1.4 to 1.5.
9. An example of nido-borane from the following is
(a) B4H10
(b) B6H10
(c) B6H12
(d) B8H14
Ans. (b)
Sol. 
Correct answer is (b)
10. The geometries of Ni(CO)4 and [NiCl4]2–, respectively are
(a) tetrahedral and square planar
(b) square planar and tetrahedral
(c) tetrahedral and tetrahedral
(d) square planar and square planar
Ans. (c)
Sol. In Ni(CO)4, Ni is present as Ni0.
Ni0 = [Ar]3d8 4s2 4p0
CO being strong field ligand pair up the s-electrons with d-electrons making the s-orbital empty for hybridisation. i.e., Ni in the presence of CO = [Ar]3d10 4s0 4p0
Thus,
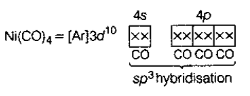
Thus, the geometry of Ni(CO)4 is tetrahedral and it is a diamagnetic complex.
Further,
in [NiCl4]2–, Ni is present as Ni2+.

Cl– being a weak field ligand is not capable to pair up the unpaired d-electrons. So, the available orbitals for it are only 4s and 4p-orbitals.
Ni in the presence of Cl– = [Ar] 3d8 4s0 4p0

Thus, it is also a tetrahedral complex.
Correct option is (c)
11. The number of S–S bonds in H2S5O6 is _________
Ans. 4
Sol. 
12. In atomic absorption spectroscopy, the atomization process utilizes
(a) flame
(b) electric field
(c) magnetic field
(d) electron beam
Ans. (a)
Sol. The atomic absorption spectroscopy, the atomization process utilizes flame (flame atomizer).
Correct option is (a)
13. At room temperature, the number of singlet resonances observed in the 1H NMR spectrum of Me3CC(O)NMe2 (N N–dimethyl pivalamide) is ________
Ans. 3
Sol. 
At room temperature

Restricted rotational three singlet
Correct answer is (3)
14. Amongst the following, the metal that does NOT form homoleptic polynuclear carbonyl is
(a) Mn
(b) Fe
(c) Cr
(d) Co
Ans. (c)
Sol. Cr metal is unable to form polynuclear metal carbonyl complexes due to steric hindrance.
Correct option is (c)
15. The reaction of [Cp2TaMe2]I (Cp = C5H5–) with NaOMe yields.
(a) [Cp2Ta(OMe)2]I
(b) [Cp2Ta(Me)OMe]I
(c) Cp2Ta(Me) = CH2
(d) Cp2Ta(OMe) = CH2
Ans. (c)
Sol. 
Correct answer is (c)
16. The complexes [Co(H2O)4Cl2]NO2 and [Co(H2O)4Cl(NO2)]Cl are
(a) linkage isomers
(b) positional isomers
(c) ionization isomers
(d) optical isomers
Ans. (c)
Sol. The given two complexes give different ions when subjected to ionisation.

Thus, these are called ionisation isomers.
Correct option is (c)
17. The major product of the following reaction is

(a) 
(b) 
(c) 
(d) 
Ans. (c)
Sol. 
Correct answer is (c)
18. Amongst the following, the structure of guanosine is
(a) 
(b) 
(c) 
(d) 
Ans. (d)
Sol. 
19. The correct order of IR stretching frequency of the C=C in the following olefins is

(a) I > II > III
(b) II > III > I
(c) III > II > I
(d) III > I > II
Ans. (c)
Sol. Increasing in the size decreasing IR (C = C) streching frequency)
(III) > (II) > I
Correct option is (c)
20. The correct order of the solvolysis for the following chlorides in acetic acid is

(a) II > I > III
(b) III > II > I
(c) III > I > II
(d) I > III > II
Ans. (b)
Sol. 
Reaction proceed through SN1 mechanism, formation of 2° carbocation.

Reaction occurs through NGP, formation of four and Six membered ring produce formation of four membered ring in less feasible which is not stable product. Also, –I effect of O-atom destabilised the carbocation.
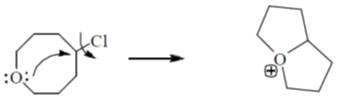
Reaction proceed through NGP formation five, five ring membered ring formation.
So correct order of solvolysis
III > II > I
Correct option is (b)
21. Formation of the product in the following photochemical reaction involves

(a)  rearrangement
rearrangement
(b) Paterno-Buchi reaction
(c) [2, 3]-sigmatropic rearrangement
(d) Norrish type I reaction
Ans. (a)
Sol. 
22. The correct order of stability for the following conformations of cyclohexane is

(a) I > II > III
(b) I > III > II
(c) II > I > III
(d) III > I > II
Ans. (b)
Sol. Twist boat form is free from flagpole interaction. Boat form is less stable than twist boat form due to presence of flagpole interaction. Half chair is less stable due to very steric occur. Half chair contain angular strain and eclipsing effect. But boat form contain 1, 4 flagpole interaction and edipsing effect, so less stable. But in twist boat form both facts decrease. So twist boat forms is more stable than boat form.

Correct option is (b)
23. The major product formed in the following reaction is

(a) 
(b) 
(c) 
(d) 
Ans. (c)
Sol. 
24. The overall yield (in %) for the following reaction sequence is _______

Ans. (57.6)
Sol. Step I Reaction (A) is 100%, react with reagent observed yield is 90%.
Step II 90% yield of B react with reagent, observed yield is 72%

Step II 72% yield react with reagent C observed yield is 57.6

Finally observed yield = 57.6
Correct option is (57.6)
25. The most suitable reagent combination to effect the following conversion is

(a) (i) NaH, CS2, then MeI; (ii) Bu3SnH, AlBN, C6H6, Reflux
(b) (i) I2, PPh3, imidazole; (ii) H2, 10% Pd-C, AcOH, high pressure
(c) (i) Me3SiCl, pyridine, DMAP; (ii) Bu3SnH, AlBN, C6H6, reflux
(d) (i) MsCl, pyridine, DMAP; (ii) LiAlH4, THF, reflux
Ans. (a)
Sol. 


Q.26 – Q.55: Carry TWO marks each.
26.  is a proposed hydrogenic wavefunction, where Z = atomic number, r = radial distance from the nucleus,
is a proposed hydrogenic wavefunction, where Z = atomic number, r = radial distance from the nucleus,  azinuthal angle, N is a constant. The INCORRECT statement about
azinuthal angle, N is a constant. The INCORRECT statement about 
(a)  in the xy-plane
in the xy-plane
(b) two radial nodes are present in 
(c) one angular node is present in 
(d) the size of the orbital decreases with increase in atomic number
Ans. (b)
Sol. 
The power of one angular node
one angular node
two radical node not prasent on eV radial node Z = r/6

The size of the orbital decrease with increase in active number
Correct option is (b)
27. The van der waals constant a and b of CO2 are 3.64 L2 bar mol–2 and 0.04 L mol–1, respectively. The value of R is 0.083 bar dm3 mol–1 K–1. if one mole of CO2 is confined to a volume of 0.15L at 300K, then the pressure (in bar) exerted by the gas, is ________.
Ans. (60 – 66)
Sol. We know that van der Waals' equation for 1 mole of a gas is

On substituting values, we get


28. A plot of osmotic pressure against concentration (gL–1) of a polymer is constructed. The slope of the plot
(a) increases with increase in temperature
(b) increases with increase in molar mass of the polymer
(c) decreases with decrease in concentration of the polymer
(d) decreases with increase in temperature.
Ans. (a)
Sol. 
The slope of the plot increases with increase in temperature
Correct option is (a)
29. A platinum electrode is immersed in a solution containing 0.1 M Fe2+ and 0.1 M Fe3+. Its potential is found to be 0.77V against SHE. Under standard conditions and considering activity coefficients to be equal to unity, the potential of the electrode, when the concentration of Fe3+ is increased to 1 M, is _____
Ans. (0.8291)
Sol. 

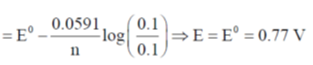


E = 0.8291V
Correct option is (0.8291).
30. Molybdenum crystallizes in a bcc structure with unit cell dimensions of 0.314 nm. considering the atomic mass of molybdenum to be 96, its density (in kg m–3) is __________
Ans. (10296.7)
Sol. 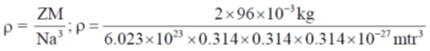

Correct answer is (10296.7).
31. The ratio of molecules distributed between two states is 9.22 × 106 at 300K. the difference in energy (in kJ mol–1) of the two states is _________.
Ans. (39.98)
Sol. 


16.03 × 1.38 × 10–25 × 3 J × 6.023 × 1023 
399.8 × 102 J = 39.98 KJ/mole
Correct answer is (39.98)
32. A Carnot engine operates at 55% efficiency. If the temperature of reject steam is 105ºC, then the absolute temperature of input steam is __________
Ans. (840)
Sol. 


33. Of the following plots, the correct representation of chemical potential  against absolute temperature (T) for a pure substance is (S, L and g denote solid, liquid and gas phases, respectively)
against absolute temperature (T) for a pure substance is (S, L and g denote solid, liquid and gas phases, respectively)
(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol. 
34. The enthalpy of fusion of ice at 273K is 6.01 kJ mol–1 and the enthalpy of vaporization of water at 273K is 44.83 kJ mol–1. The enthalpy of sublimation (in kJ mol–1) of ice at 273K, is ________
Ans. (50.84)
Sol. 
= 6.01 + 44.83

35. Suppose  are two hybrid orbitals:
are two hybrid orbitals:


The angle (in degrees) between them is ________
Ans. (179)
Sol. 



36. BCl3 and NH4Cl were heated at 140ºC to give compound X, which when treated with NaBH4 gave another compound Y. Compounds X and Y are
(a) X = B3N3H3Cl3 and Y = B3N3H6
(b) X = B3N3H9Cl3 and Y = B3N3H6
(c) X = B3N3H3Cl3 and Y = B3N3H12
(d) X = B3N3Cl6 and Y = B3N3H6
Ans. (a)
Sol. 
37. The number of microstates in term 1G is
(a) 13
(b) 11
(c) 9
(d) 7
Ans. (c)
Sol. (2S + 1) = 1 S = 0
l = 4[for G]
Microstate = (2S + 1) (2l + 1)
1 × (2 × 4 + 1) = (9)
Correct option is (c)
38. The set of protons (underlined) in CH3CH2CH2OCH3 that would exhibit different splitting patterns in high (500 MHz) and low (60 MHz) field 1H NMR, is
(a) 
(b) 
(c) 
(d) 
Ans. (b)
Sol. 
The underlined proton shows different signal in NMR at 500 MHz and 60 mHz
Correct option is (b)
39. Amongst the following, the complex ion that would show strong Jahn-Teller distortion is
(a) [Cr(H2O)6]2+
(b) [Ti(H2O)6]3+
(c) [Co(H2O)6]2+
(d) [Fe(H2O)6]2+
Ans. (a)
Sol. The compounds having unsymmetrically filled eg orbitals show strong J.T.D.


40. Amongst the following, the metal carbonyl species having the highest  stretching frequency is
stretching frequency is
(a) [Mn(CO)6]+
(b) Cr(CO)6
(c) [V(CO)6]–
(d) [Fe(CO)4]2–
Ans. (a)
Sol. The  stretching frequency
stretching frequency  bond order
bond order  positive oxidation state.
positive oxidation state.
Or we can say greater the negative charge on metal greater will be  back bonding and hence lesser will be new CO.
back bonding and hence lesser will be new CO.
Correct option is (a)
41. The correct order of thermal stability for the given compounds is
(a) TiMe4 > Ti(CH2CMe3)4 > TiEt4
(b) TiEt4 > Ti(CH2CMe3)4 > TiMe4
(c) TiMe4 > TiEt4 > Ti(CH2CMe3)4
(d) Ti(CH2CMe3)4 > TiMe4 > TiEt4
Ans. (d)
Sol. The stability of compound decreases due to presence of  Transition metal possess vaccant vaccant d-orbital which involve in kinetically facile reaction such as
Transition metal possess vaccant vaccant d-orbital which involve in kinetically facile reaction such as 
The driving force for  elimination reaction is the formation of stronger M – H bond than M-alkyl bond.
elimination reaction is the formation of stronger M – H bond than M-alkyl bond.
Correct option is (d)
42. Amongst the following, the complex ion that is expected to show the highest magnetic moment at room temperature is
(a) [Ni(CN)4]2–
(b) [Fe(CN)6]3–
(c) [Cu(H2O)6]2+
(d) [Co(CN)6]3–
Ans. (b)
Sol.  dsp2-square planar zero unpaired electron.
dsp2-square planar zero unpaired electron.

unpaired electron is one and t2g is unsymmetrically filled so orbital contribution.


43. MnCr2O4 is
(a) normal spinel with total CFSE of –15.5 Dq
(b) inverse spinel with total CFSE of –15.5 Dq
(c) normal spinel with total CFSE of –24 Dq
(d) inverse spinel with total CFSE of –24 Dq
Ans. (c)
Sol. MnCr2O4 is normal spinel because Mn2+ having CFSE less than Cr3+ in Oh site.
Total CFSE = CFSE of Mn2+ + 2CFSE of Cr3+

Correct option is (c)
44. Mg2+ is preferred in photosynthesis by chlorphyll because
(a) it has strong spin-orbit coupling
(b) it has weak spin-orbit coupling
(c) it is a heavy metal
(d) it binds strongly with chlorophyll
Ans. (d)
Sol. In photosynthesis, Mg2+ present in chlorophyll. Because it binds strongly with chlorophyll.
Correct option is (d)
45. In Monsanto acetic acid process shown below, the role of HI is

(a) to convert CH3OH to a stronger nucleophile (CH3O–)
(b) to reduce the Rh(I) catalyst to a Rh(0) species
(c) to reduce a Rh(III) active species to a Rh(I) species in the catalytic cycle
(d) to convert CH3OH to CH3I
Ans. (d)
Sol. In monsanto acetic and process role of HI is to convert CH3OH to CH3I

46. Formation of the ketone H from the diazoketone I involves

(a) generation of carbene and a [2, 3]-sigmatropic rearrangement
(b) generation of carbene and an electrocyclic ring closing reaction
(c) generation of ketene and a [2+2] cycloaddition
(d) generation of ketene and a [3, 3] sigmatropic rearrangement
Ans. (d)
Sol. 

47. The major products X and Y formed in the following reaction sequence are

(a) 
(b) 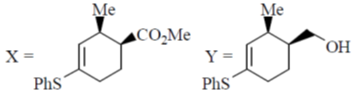
(c) 
(d) 
Ans. (a)
Sol. 
Correct answer is (a)
48. The major products X and Y formed in the following reactions are

(a) 
(b) 
(c) 
(d) 
Ans. (b)
Sol. 
49. The major product X and Y formed in the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol. 
Correct answer is (a)
50. The product of the following reaction gave 6 line 13C NMR spectrum with peaks at  46, 37, 33 ppm. The structure of the product is
46, 37, 33 ppm. The structure of the product is

(a) 
(b) 
(c) 
(d) 
Ans. (c)
Sol. 
Correct answer is (c)
51. The major product formed in the following reaction is

(a) 
(b) 
(c) 
(d) 
Ans. (c)
Sol. 
Correct answer is (c)
52. The major products X and Y formed in the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. (a)
Sol. 

Correct answer is (a)
53. The major products X and Y formed in the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. (b)
Sol. 
Correct answer is (b)
54. Given the fact that 1, 3-butadiene has a UV absorption of 217nm, the absorption wavelength (in nm) for the conjugated system shown below is ______________

(Use these absorption value for auxochromic groups:
alkyl: +5; exo-cyclic double bond: +5; every additional conjugated C = C : + 30)
Ans. (282)
Sol. 

Correct answer is (282)
55. The m/z value of the detectable fragment formed by McLafferty like rearrangement of the following compound in mass spectrometer is ___________

Ans. (41)
Sol. 
Correct option is (41)
