CSIR NET CHEMISTRY (Dec-2018)
Previous Year Question Paper with Solution.
21. The correct order of acceptor ability of the phosporus ligands is
(a) 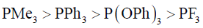
(b) 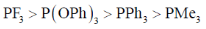
(c) 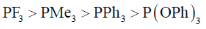
(d) 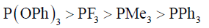
Ans. b
Sol. As the electronegativity of X in PX3 increases, π-acceptor tendency increases,
PF3 > P(OPh)3 > PPh3 > PMe3
Correct option is (b)
22. In the 31P{1H} NMR spectrum of a diamagnetic complex mer-[M(PR3)3Cl3] (M = transition metal, I = 0) expected number of resonance(s) is
(a) Three
(b) One
(c) Two
(d) Six
Ans. c
Sol. 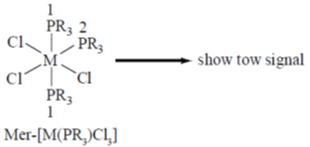
Correct answer is (c)
23. Consider the species NO, I2, I–2, Cu2+ and VO2+. The number of paramagnetic species among them and the EPR inactive species, respectively, are
(a) 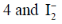
(b) 4 and I2
(c) 3 and VO2+
(d) 3 and NO, Cu2+
Ans. b
Sol.  is paramagnetic. Hence, EPR active where as I2 is diamagnetic and EPR inactive.
is paramagnetic. Hence, EPR active where as I2 is diamagnetic and EPR inactive.
Correct answer is (b)
24. Identify the correct statement(s) for H3B.CO.
(A) sp2 hybridized orbital of B accepts the lone pair of CO.
(B) Its vCO value is more than that for free CO
(C) Formal oxidation state of C is +4 in the compound
Answer is
(a) A and B
(b) B only
(c) A only
(d) A and C
Ans. b
Sol. 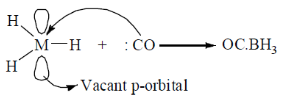
In BH3.CO formation CO interact with vaccant p-orbital, where as electron is donated by antibonding orbital of CO due to which bond order increases and vCO increases.
Correct option is (b)
25. Match the items of Column-I with those of Column-II
Column-I Column-II
(A) Laser source (I) Electron Capture Detector
(B) Thermometric titration (II) Polarography
(C) Gelatin (III) Heat of reaction
(D) Gas-liquid chromatography (IV) Spectrofluorimetry
Correct Answer is
(a) A-IV, B-III, C-II, D-I
(b) A-I, B-III, C-II, D-IV
(c) A-IV, B-II, C-III, D-I
(d) A-III, B-II, C-IV, D-I
Ans. a
Sol. Thermometric titration is the method in which a titrant is added stepwise to a vessel containing another reactant. The enthalpy change on heat of reaction causes a temperature change which when plotted versus volume of titrant is used to detect the end point.
(i) Electron capture detector is a device for detecting atoms and molecules in a gas and is used in atoms and molecules in a gas and is used in Gas-liquid chromatography.
(ii) The abnormal peak which appears in polographic titration can be supposed by addition of Gelatin.
(iii) In spectrofluorimetry laser may used as source.
Correct answer is (a)
26. Consider compounds PF5, SbF5, PH3 and SbH3. The strongest acid and the strongest base among these are, respectively.
(a) PF5 and PH3
(b) SbF5 and PH3
(c) SbF3 and SbH3
(d) PF3 and SbH3
Ans. b
Sol. SbF5 is strongest acid and PH3 is strongest base.
Correct answer is (b)
27. Among SiCl4, P(O)Cl3, NF3, trans-[SnCl4(py)2] (py = pyridine), those with zero dipole moment are
(a) SiCl4 and NF3
(b) SiCl4, P(O)Cl3 and trans-SnCl4(py)2
(c) SiCl4 nad trans-SnCl4(py)2
(d) NF3 and trans-SnCl4(py)2
Ans. c
Sol. 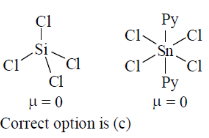
28. The standard reduction potentials in acid medium for F2, Cl2, Na and Zn are in the order
(a) 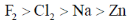
(b) 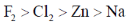
(c) 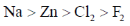
(d) 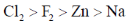
Ans. b
Sol. The standard reduction potential in acid follow the order
F2 > Cl2 > Zn > Na
Correct answer is (b)
29. The characters of LUMO and CN– and O2 respectively, are
(a) 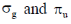
(b) 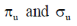
(c) 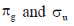
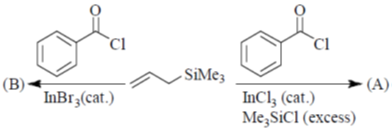
(d) 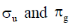
Ans. c
Sol. According to MOT the LUMO of CN– and O2 respectively are 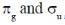
Correct answer is (c)
30. The intermediate 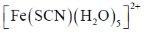 is decided in the reaction of
is decided in the reaction of 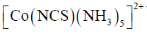 with
with 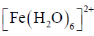 in aqueous medium to produce
in aqueous medium to produce 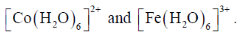
The mechanism of the reaction is
(a) Interchange dissociateve
(b) Interchange associative
(c) Inner sphere electron transfer
(d) Outer sphere electron transfer
Ans. c
Sol. 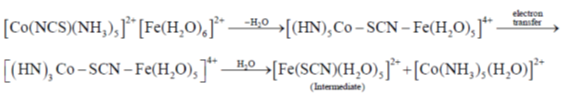
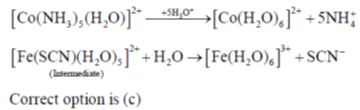
31. The chelate rings made by macrocyclic ligand in vitamin B12 are
(a) One five-membered and three six-membered
(b) Two five-membered and two six-membered
(c) Three five-membered and one six-membered
(d) Four six-membered
Ans. a
Sol. 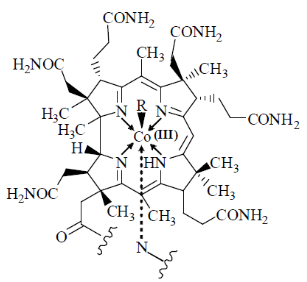
Correct answer is (a)
32. For magnesium complex of EDTA2–, the number of N-donor and O-donor centers, are respectively
(a) Two and four
(b) two and two
(c) two and six
(d) two and eight
Ans. a
Sol. 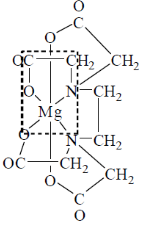
Correct answer is (a)
33. The correct set of electronic configurations for metal ions in octahedral coordination geometry for strong Jahn-Teller distrotion is
(a) 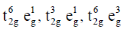
(b) 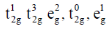
(c) 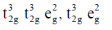
(d) 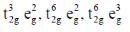
Ans. a
Sol. For strong Jahn-Teller distortion eg orbital must be assymetrical filled.
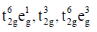
Correct answer is (a)
34. The order of relative rate of cyclization of following bromocarboxylates to generate corresponding lactones is
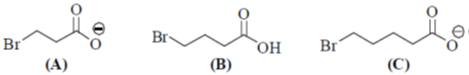
(a) B > A > C
(b) A > C > B
(c) B > C > A
(d) C > B > A
Ans. c
Sol. The rate of cyclization is a function of ring size and the relative order is 5 > 6 > 4
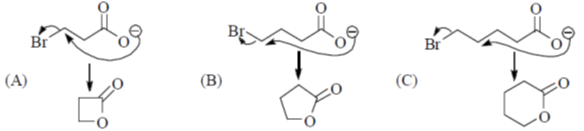
Thus, order of lactonization will be B > C > A
Correct option is (c)
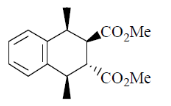
35. Oxidation of A with HNO3/H2O provides the product(s) which is (are)
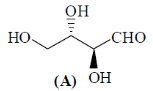
(a) Optically inactive as it is recemic mixture
(b) Optically inactive as it is meso
(c) Optically active as it is a single diastereomer
(d) Optically active as it s a single enantiomer
Ans. b
Sol. 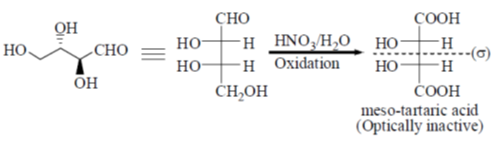
Correct answer is (b)
36. The major product formed in the following reaction is
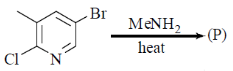
(a) 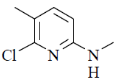
(b) 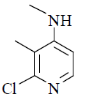
(c) 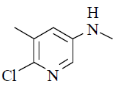
(d) 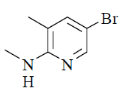
Ans. d
Sol. 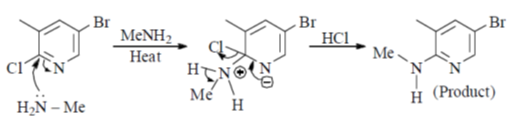
Nucleophilic attack is more facile at 2-and 4-position of pyridine ring. Here, Cl-atom is present at 2-position as leaving group.
Correct answer is (d)
37. The order of reactivity of the following dienes towards Diels-alder reaction is –
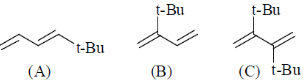
(a) B > A > C
(b) A > C > B
(c) B > C > A
(d) C > B > A
Ans. a
Sol. The diene must be capable of achieveing s-cis conformation for the Diels-Addler reaction.
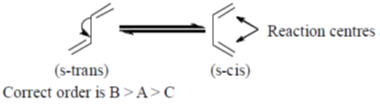
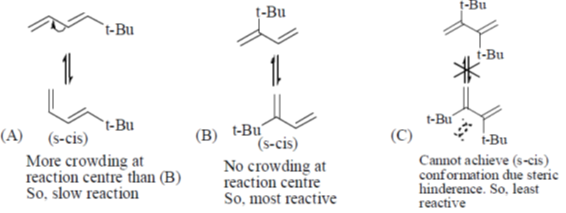
Correct answer is (a)
38. Among the following, the optically active compound is
(a) 
(b) 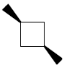
(c) 
(d) 
Ans. d
Sol. 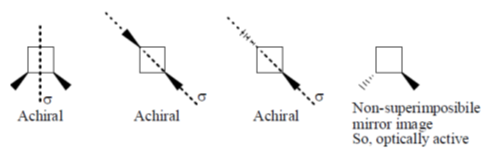
Correct answer is (d)
39. Among the following reaction(s) which provide(s) 1-butanes as the major product is (are)
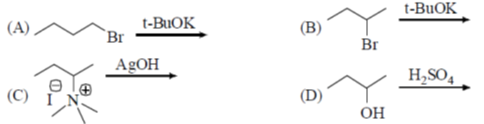
(a) A alone
(b) A and B
(c) A and C
(d) C and D
Ans. c
Sol. A and C gives 1-butene, B and D gives 2-butene.

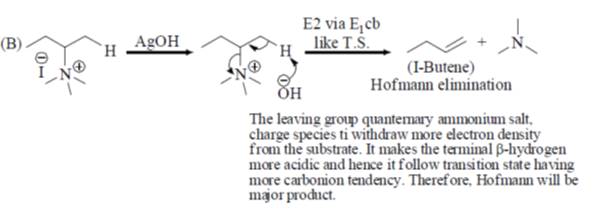
Correct answer is (c)
40. The major product formed in the following reaction is
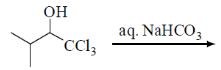
(a) 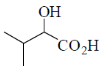
(b) 
(c) 
(d) 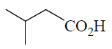
Ans. a
Sol. Alkaline hydrolysis of trichloro alkane gives corresponding carboxylic acid
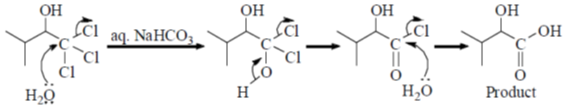
Correct answer is (a)
41. Among the following, the compound that will have highest rate for nucleophilic substitution through SN1 mechanism is
(a) 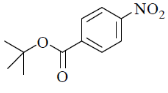
(b) 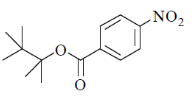
(c) 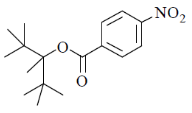
(d) 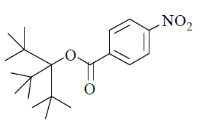
Ans. d
Sol. 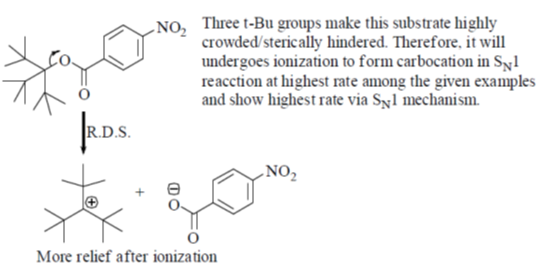
Correct answer is (d)
42. The major product formed in the following reaction is
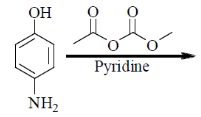
(a) 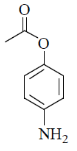
(b) 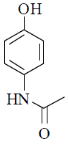
(c) 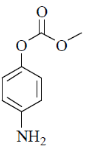
(d) 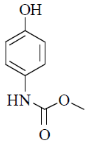
Ans. b
Sol. Mixed anhydride as acylating agent
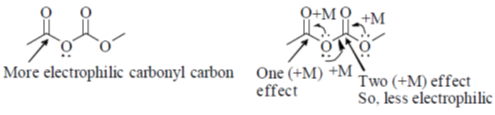
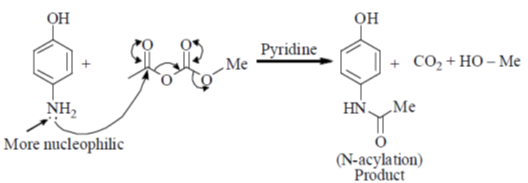
Correct answer is (b)
43. The mechanism of acid catalyzed hydrolysis of benzonitrile involes
(a) 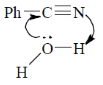
(b) 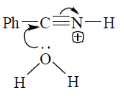
(c) 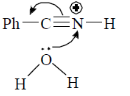
(d) 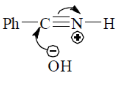
Ans. b
Sol. 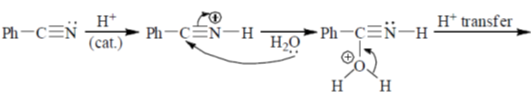
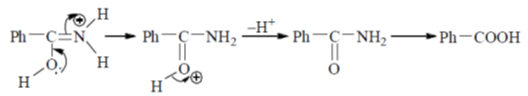
Correct answer is (b)
44. In the energy profile diagram of the reaction given below, the species A would correspond to the position.
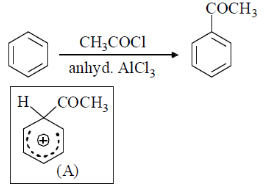
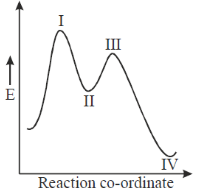
(a) I
(b) II
(c) III
(d) IV
Ans. b
Sol. 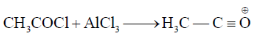
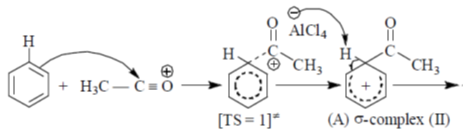
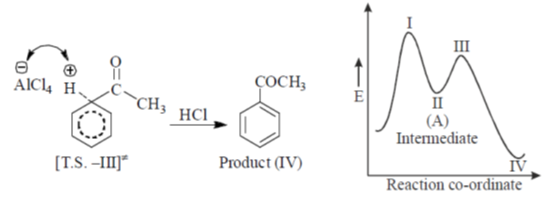
Correct answer is (b)
45. Following reaction is an example of
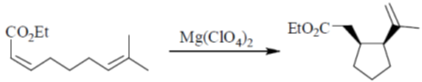
(a) Alder-Ene reaction
(b) Michael addition
(c) Sigmatropic Rearrangement
(d) Wagner-Meerwein Rearrangement
Ans. a
Sol. 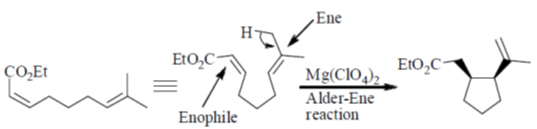
Presence of Lewis acid Mg(ClO4)2 makes the reaction more favourable.
Correct option is (a)
46. In IR spectra, the stretching frequency (in cm–1) of the carbonyl group of the following compound is in the order
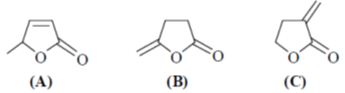
(a) B > A > C
(b) A > C > B
(c) B > C > A
(d) C > B > A
Ans. c
Sol. 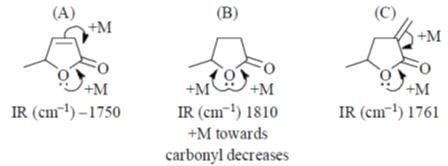
Correct order is B > C > A
Correct option is (c)
47. The uncertainty in the position of a moving electron is 100 pm. The uncertainty in its speed is closest to (me = 9.11×10–31 kg; h = 6.63×10–34 J.s)
(a) 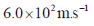
(b) 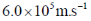
(c) 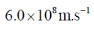
(d) 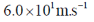
Ans. b
Sol. Accroding to Heisenberg's uncertainty principle,
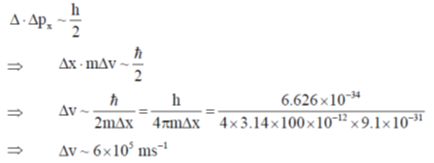
Correct answer is (b)
48. The spectrum of sodium atom has a closely separated dublet at 16956.2 and 16973.4 cm–1. The higher energy transition is due to
(a) 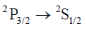
(b) 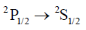
(c) 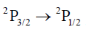
(d) 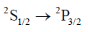
Ans. a
Sol. The correct involed in the transition are 3s and 3p. The erergy of 3s is lower than that of 3p. The 3p orbital energy level gets split into two further energy level i.e. 3p1/2 and 3p3/2, out of which 3p3/2 is of higher than 3p1/2. Therefore, in emission spectrum the large value of transition should be because of transition between 3p3/2 – 3s1/2. (We have used before orbital because we are denoting the orbital which is involed in transition. In question instead of 3p3/2 they have written 3p3/2 – 2st/2. They are mentioning spin multiplicity rather than orbital).
Correct answer is (a)
49. N2O molecule belongs to the point group
(a) 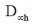
(b) 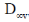
(c) 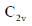
(d) 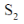
Ans. b
Sol. 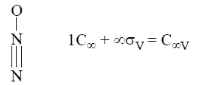
Correct answer is (b)
50. For a closed system in the absence of non-PV work, the correct statement is
(a) dU = TdS – PdV
(b) dG = VdP + SdT
(c) dU = TdS + PdV
(d) dU = VdP – SdT
Ans. a
Sol. For the closed system the correct statement is dU = TdS – PdV (Maxwell equation)
Correct answer is (a)
51. The volume change in a certain phase transition is 2.0 mL mol–1 at the transition point. From this, we may conclude that the transition is most likely to
(a) first order phase transition
(b) second order phase transition
(c) third order phase transition
(d) 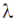 phase transition
phase transition
Ans. a
Sol. Condition of first order phase transition is
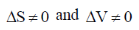
Correct answer is (a)
52. Root mean square speed of the molecules of a perfect gas is proportional to
(a) 1/T1/2
(b) T
(c) T1/2
(d) 1/T
Ans. c
Sol. 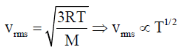
Correct answer is (c)
53. For a second-order reaction, the straight line among the following plots is
(a) [X] versus time
(b) 1/[X] versus time
(c) log[X] versus 1/time
(d) log[X] versus time
Ans. b
Sol. For second order reaction
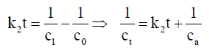
Correct answer is (b)
54. The activation energy of a reaction reduces by 12 kcal mol–1 in the presence of an enzyme at 300 K. Assuming pseudo-first order kinetics, calculate the factor by which the reaction rate is increased. [Given: R = 2 cal K–1mol–1]
(a) 2 × 10–9
(b) 1.02
(c) 8.7 × 106
(d) 5 × 108
Ans. d
Sol. 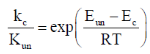 (where un = uncatalysed reaction, c = catalysed reaction)
(where un = uncatalysed reaction, c = catalysed reaction)
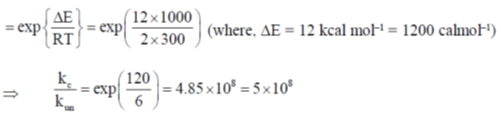
Correct answer is (d)
55. The correct statement among the following is
(a) Salt bridge is required for the mixing of the solution in the two half cells.
(b) Salt bridge allows current to flow between the half cells without mixing the solutions
(c) Salt bridge enhances the rate of the reaction
(d) Salt bridge consists of a non-electrolyte in a gel.
Ans. b
Sol. Correct Statement: Salt bridge allows current to flow between the half cells with out mixing the solution.
Correct answer is (b)
56. The standard free energy of the reaction
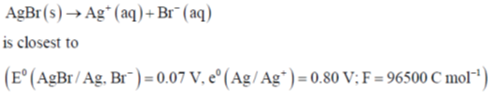
(a) 7 kJ mol–1
(b) 70 J mol–1
(c) 70 kJ mol–1
(d) 7 J mol–1
Ans. c
Sol. The given cel, 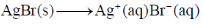 can written as
can written as
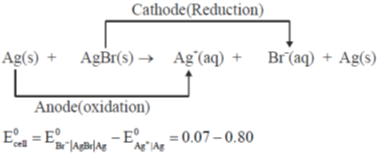
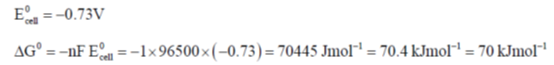
Correct answer is (c)
57. The internal pressure of a liquid drop (radius = 10–6 m) is greater than the external pressure by 1.5 × 105 Nm–2. The surface tension (mN m–1) of the liquid is closest to
(a) 150
(b) 125
(c) 100
(d) 75
Ans. d
Sol. 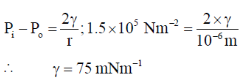
Correct answer is (d)
58. In a cubic crystal, the (1 1 1) and (222) reflections are observed, but not the (001) reflection. The Bravais lattice is
(a) body centred cubic
(b) face centred cubic
(c) simple cubic
(d) side centred cubic
Ans. b
Sol. 
Correct answer is (b)
59. The dispersity of a polymeric sample is
(a) 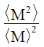
(b) 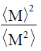
(c) 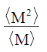
(d) 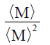
Ans. a
Sol. The polydispersity can be expressed in terms of the normalised standard deviation.
Therefore, polydispersity 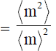
Correct answer is (a)
60. The keto-hexose among the following is
(a) Xylose
(b) Galactose
(c) Fructose
(d) Mannose
Ans. c
Sol. 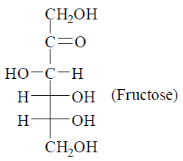
Among given compound fructose is the only one which contains a ketone functional group and having six carbon stom, thus ketohexose.
Correct answer is (c)
61. The product for the reaction given below is
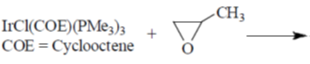
(a) 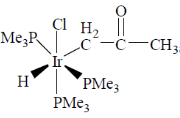
(b) 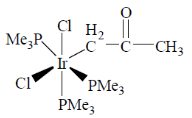
(c) 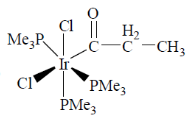
(d) 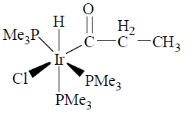
Ans. b
Sol. 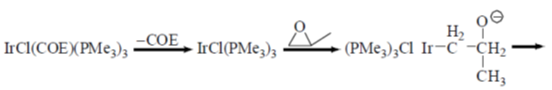
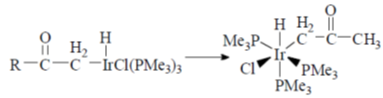
Correct answer is (b)
62. The 13P {1H} NMR spectrum of cis-[Pt(PEt3)2Cl2] (195Pt(33.8% abundance) I = ½ its other isotopes are NMR inactive; 31P : I = ½) is comprised with satellite peaks of a
(a) triplet
(b) singlet
(c) doublet
(d) quartlet
Ans. b
Sol. 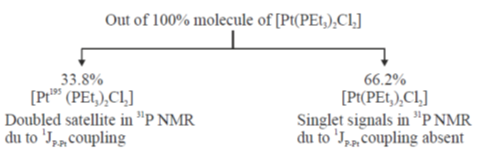
Overall the 31P NMR signal will be singlet signal with 33.8% doublet satellite as given below.
The 31P {1H} NMR spectrum is comperised with satellite peak of a singlet signal.
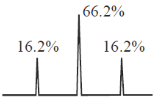
Correct answer is (b)
63. The correct order of intensity of the d-d transitions in the complexes of a 3d-transition metal ion M2+ is
(a) cis–[M(H2O)4Cl2] > trans – [M(H2O)4Cl2] > [M(H2O)6]2+
(b) [M(H2O)6]2+ > cis – [M(H2O)4Cl2] > trans – [M(H2O)4Cl2]
(c) trans – [M(H2O)4Cl2] > cis – [M(H2O)4Cl2] > [M(H2O)6]2+
(d) [M(H2O)6]2+ > cis – [M(H2O)4Cl2] ≈ trans – [M(H2O)4Cl2]
Ans. a
Sol. The correct order or intensity of d-d transitions in the complexes follows order
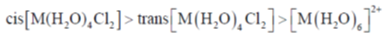
Correct answer is (a)
64. The reaction of decaborane B10H14 with acetylene in the presence of Et2S gives
(a) C2B10H12
(b) C2B8H10
(c) C2B10H14
(d) C2B9H11
Ans. a
Sol. 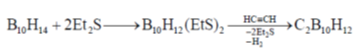
Correct answer is (a)
65. In compound N3P3F6, the geometry around nitrogen and phosphorus, respectively are
(a) pyramidal and tetrahedral
(b) planar and tetrahedral
(c) pyramidal and planar
(d) planar and trigonal bipyramidal
Ans. b
Sol. 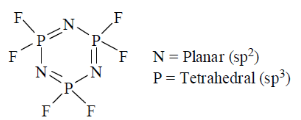
Correct answer is (b)
66. The number of 2c-2e bonds ('x') of a molecule is related to 'N' (valence electrons) and 'n' (skeletal atoms) by x = (8n – N)/2. For P4S3, the values of x, N and n, respectively, are
(a) 7, 38, 9
(b) 7, 24, 9
(c) 9, 38, 7
(d) 9, 24, 7
Ans. c
Sol. 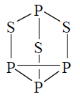
Valence electron (N) 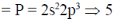
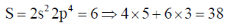
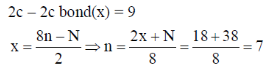
Correct answer is (c)
67. Match the following complxes with their νCO stretching frequency
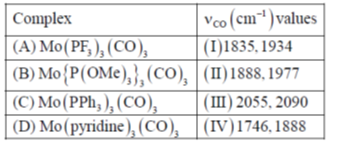
The correct match is
(a) A-I, B-IV, C-II, D-III
(b) A-III, B-II, C-I, D-IV
(c) A-IV, B-III, C-I, D-II
(d) A-I, B-II, C-III, D-IV
Ans. b
Sol. Complex:
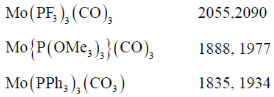
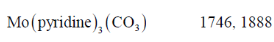
 tendency of spectator ligand is
tendency of spectator ligand is  Therefore, PF3 reduce more electron density from the metal in complex to pyridine consequently. As the electron density on metal decreases, M–C bond strength decreases, C–O bond strength increases. Hence, vCO increases.
Therefore, PF3 reduce more electron density from the metal in complex to pyridine consequently. As the electron density on metal decreases, M–C bond strength decreases, C–O bond strength increases. Hence, vCO increases.
Correct answer is (b)
68. The 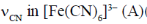 and [Fe(CN)6]4– (B) and
and [Fe(CN)6]4– (B) and 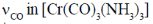 (C) and [Cr(CO)6] (D) are compared below. The pair with correct order is
(C) and [Cr(CO)6] (D) are compared below. The pair with correct order is
(a) A > B; C > D
(b) A > B; C < D
(c) A < B; C > D
(d) A < B; C < D
Ans. b
Sol. 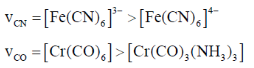
Correct answer is (b)
69. Consider the following statements for [FeO4]2–
(A) It is stable in the pH range 0–14
(B) It is stable in strongly basic medium only
(C) It is a very strong oxidizing agent
(D) The isomer shift in its Mossbauer spectrum is more negative compared to that of FeCl3.
The correct statements are
(a) A, C and D
(b) B, C and D
(c) B and C
(d) C and D
Ans. b
Sol. 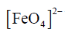
• It is stable in strongly basic medium
• Power oxdizing agent
• The isomer shift of its Mossbauer spectrum is more negative compared to that of FeCl3.
Correct answer is (d)
70. 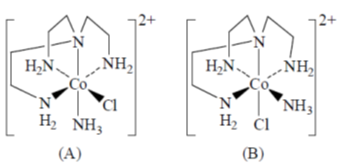
The isomers A and B undergo base hydrolysis by forming a trigonal bipyramidal intermediate. The correct statement is
(a) A reacts faster than B and both results in a mixture of products
(b) B reacts faster than A and both results in a mixture of products
(c) A reacts faster than B and B results in a mixture of products
(d) B reacts faster than A and A results in a mixture of products.
Ans. c
Sol. 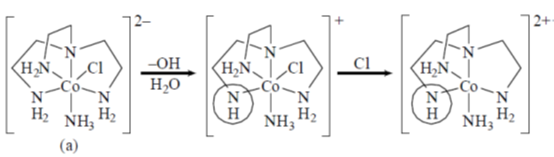
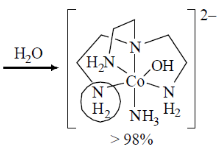
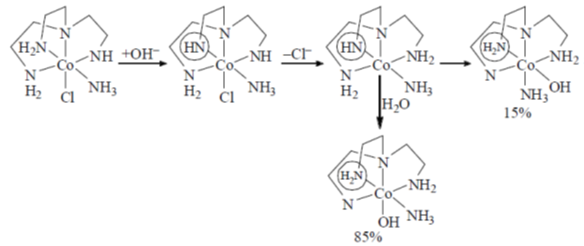
A react faster than B and B resuslt in a mixture of product.
Correct option is (c)
71. B2H6 reacts with
(A) water to give boric acid and H2O2 (B) oxygen to give B2O3 and H2
(C) water to give boric acid and H2O (D) oxygen to give B2O3 and H2O
Correct statements from the above are
(a) A and B
(b) A and D
(c) B and C
(d) B and D
Ans. d
Sol. 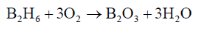
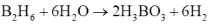
Correct answer is (d)
72. The ligand that binds strongly to the nickel center in (2, 2'-bipyridine) Ni(0) complex is
(a) 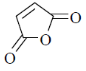
(b) 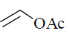
(c) 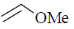
(d) 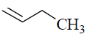
Ans. a
Sol. The ligand which have strong electron withdrawing group have strong 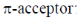 tendency. So, binds strongly
tendency. So, binds strongly
Correct option is (a)
73. Match the items given in Column-I with those given in Column-II
Column-I Column-II
(A) Magic number (I) Nuclear fission
(B) Liquid drop model of nucleus (II) Q-value
(C) Actinides (III) Radioactivity
(D) Threshold energy (IV) Shell model of nucleus
The correct match is
(a) A-IV, B-I, C-III, D-II
(b) A-II, B-I, C-III, D-IV
(c) A-III, B-IV, C-I, D-II
(d) A-IV, B-III, C-I, D-II
Ans. a
Sol. Magic number  shell model of nucleus
shell model of nucleus
Liquid drop model of nucleus  nuclear fission
nuclear fission
Actinides  radioactivity
radioactivity
Correct answer is (a)
74. The cluster type and geometry of the species [Rh9P(CO)21]2– are
(a) closo, tricapped trigonal prism
(b) arachno, trigonal prism
(c) nido, capped square antiprism
(d) nido, bicapped, trigonal prism
Ans.
Sol. 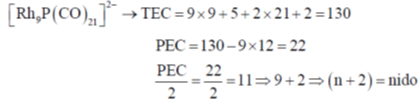
75. Hydroformylation of 1-propene with [HRh(CO)L2] leads to linear and branched formylated products. The linear hydroformylated product is formed with highest selectivity when 'L' in the rhodium complex is
(a) 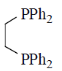
(b) 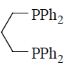
(c) 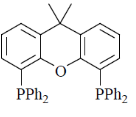
(d) 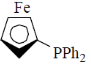
Ans. c
Sol. 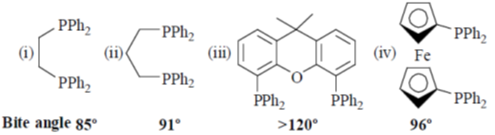
The linear and branches formylated product is depends upon natural bite angle of biphosphines. As the natural bite angle increases selectivity for linear product increases.
Correct option is (c)
76. The hydrocarbon having an analogous structures to that of P4O6 is
(a) 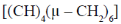
(b) 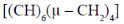
(c) 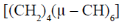
(d) 
Ans. a
Sol. 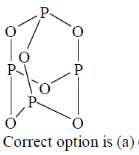
77. Match the items given below in the three columns:

Correct matches:
(a) A-III-Z, B-I-Y, C-II-X
(b) A-II-Y, B-I-X, C-III-Z
(c) A-III-Y, B-I-Z, C-II-X
(d) A-I-X, B-II-Y, C-III-Z
Ans. a
Sol. 
78. A solid sample of Na[Fe(EDTA)(H2O)s] (X) showed 5.6% weight loss at 120ºC in a thermogravimetric experiment. Identify the complex left after this weight loss.
(a) Na[FE(EDTA)(H2O)]
(b) Na[Fe(EDTA)]
(c) Na[Fe(EDTA)(H2O)2]
(d) Na[Fe(EDTA)(H2O)3]
Ans. b
Sol. n can either be 1 or 2
Molecular mass of the complex (when n = 1)
22.98 + 55.845 + 292.24 + 18.01 = 389.075 g/mol
Molecular mass after 9.6% weight loss = 367.295 which is molecular

79. Consider the two sets of molecules.
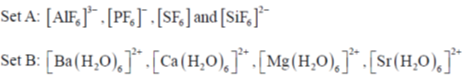
The slowest ligand exchange rate in Set A and Set B are, respectively
(a) 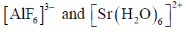
(b) 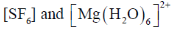
(c) 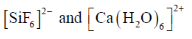
(d) 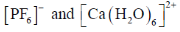
Ans. b
Sol. As the oxidation state increases and size of central decreases ligand exchange rate decreases.
Correct answer is (b)
80. Consider following statements for Eu3+
(A) The positions of sharp bands in UV-vis spectra of its complexes depend heavily on the ligand environment
(B) Its ground state term symbol is 7F0
(C) The observed magnetic moment is due to populated higher J level
(D) At 2 K its magnetic moment approaches to zero
The set of correct statements is
(a) A, C and D
(b) B, C and D
(c) A, B and D
(d) A, B and C
Ans. b
Sol. 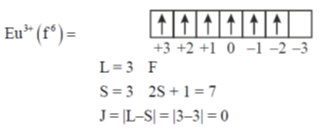
Hecnce, 7F0
Since, J = 0 hence 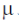 should be zero but due to population of higher J level
should be zero but due to population of higher J level  But as temperature decreases population is excited J level decreases hence magnetic moment approaches to calculated value i.e. zero.
But as temperature decreases population is excited J level decreases hence magnetic moment approaches to calculated value i.e. zero.
Correct option is (b)
81. The major product formed in th following reaction is
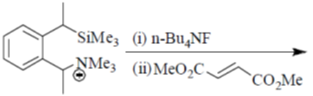
(a) 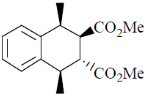
(b) 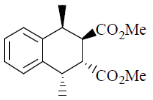
(c) 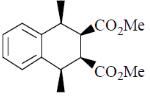
(d) 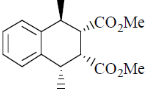
Ans. a
Sol. 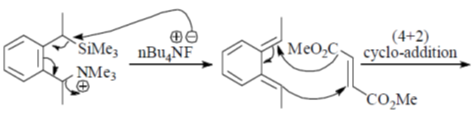
Correct answer is (a)
82. Structure of the compound displaying following characteristics spectral data
IR : 1720 cm–1
1H NMR : 6.2(br s, 1H), 5.5(br, s, 1H), 4.2(q, 2H), 2.0(s, 3H), 1.1(t, 3H) is
(a) 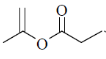
(b) 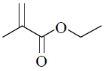
(c) 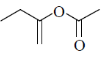
(d) 
Ans. b
Sol. IR value at 1720 cm–1 indicates the 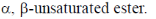
Correct answer is (b)
83. The major product formed in the following reaction is
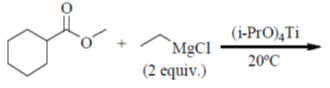
(a) 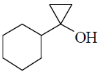
(b) 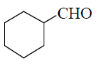
(c) 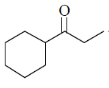
(d) 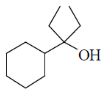
Ans.
Sol. Kulnkovich Reaction (Cyclopropanation):
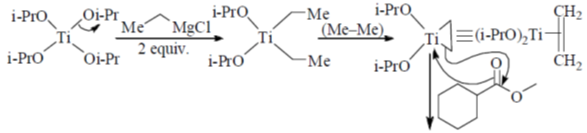
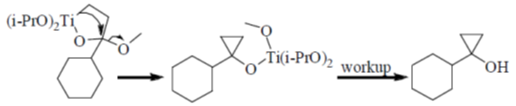
Correct answer is (a)
84. The intermediate(s) involved in the following reaction is(are)
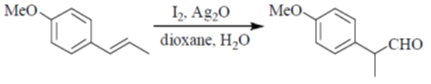
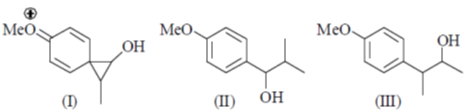
(a) Only I
(b) Only II
(c) I and II only
(d) I and III only
Ans. c
Sol. Aqueous Ag2O gives OH– ions and Ag+ ions reaction mixture

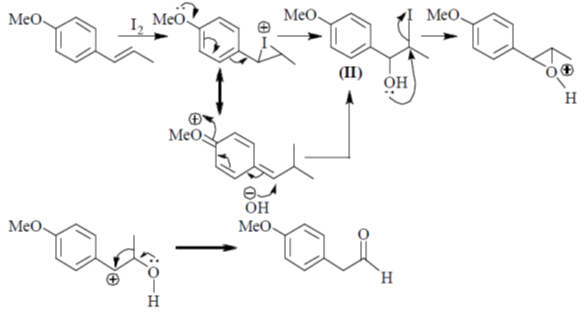
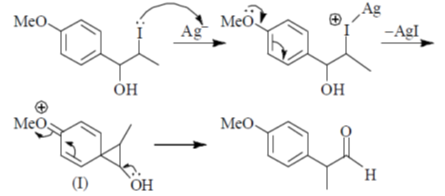
Correct answer is (c)
85. Correct sequence of reagents to be used for the following conversion is
(a) (I) NaH, 1-fluoronaphthalene; (II) NaBH4; (III) (i) (CH2O)n, Me2NH.HCl; (ii) 5 N NaOH
(b) (I) NaBH4; (II) NaH, 1-fluoronaphthalene; (III) (i) (CH2O)n, Me2NH.HCl (ii) 5 N NaOH
(c) (I) (i) (CH2O)n, Me2NH.HCl; (ii) 5N NaOH; (II) NaBH4; (III) NaH, 1-fluoronaphthalene
(d) (I) (i) (CH2O)n, Me2NH.HCl; (ii) 5N NaOH; (II) NaH, 1-fluoronaphthalene; (III) NaBH4.
Ans. c
Sol. 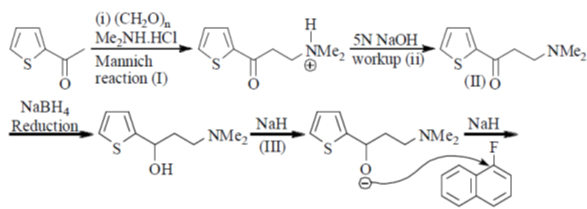
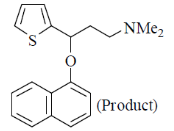
Correct answer is (c)
86. The major products (A) and (B) formed in the following reactions are
(a) 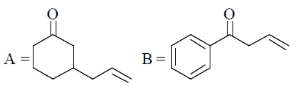
(b) 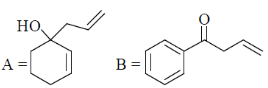
(c) 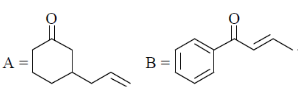
(d) 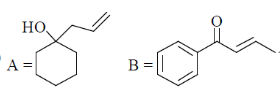
Ans. a
Sol. Presence of Lewis acid favoured the Michael 1, 4-addition reaction in enones.

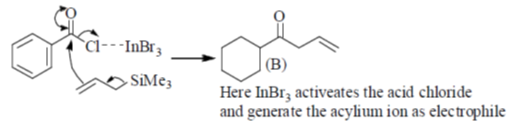
Correct answer is (a)
87. The major product formed in the following reaction is
(a) 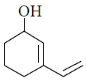
(b) 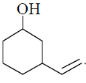
(c) 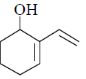
(d) 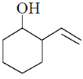
Ans. a
Sol. 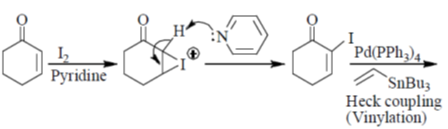
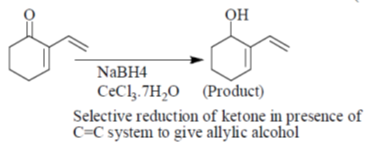
Correct answer is (a)
88. The major product formed in the following reaction is
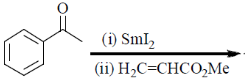
(a) 
(b) 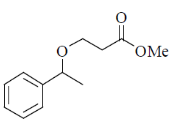
(c) 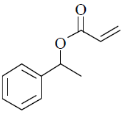
(d) 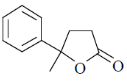
Ans. d
Sol. 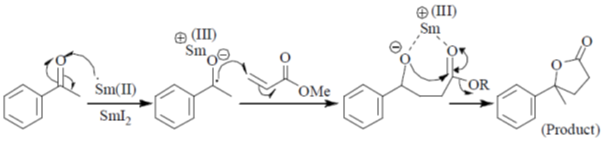
Correct answer is (d)
89. The major product formed in the following reaction is
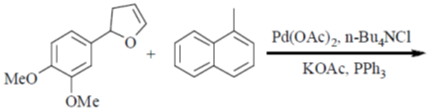
(a) 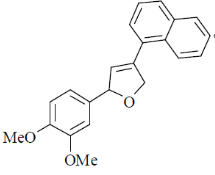
(b) 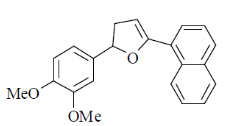
(c) 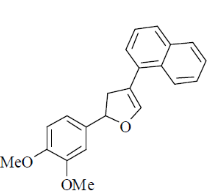
(d) 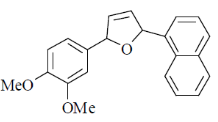
Ans. d
Sol. 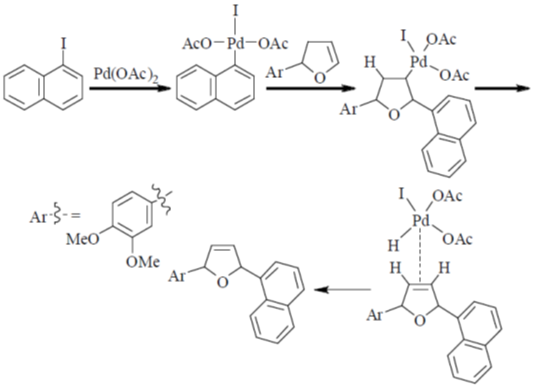
Correct answer is (d)
90. The major product formed in the following photochemical reaction is
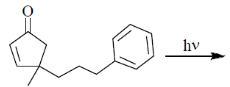
(a) 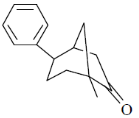
(b) 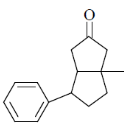
(c) 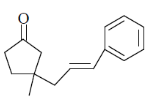
(d) 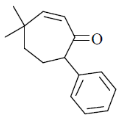
Ans. c
Sol. 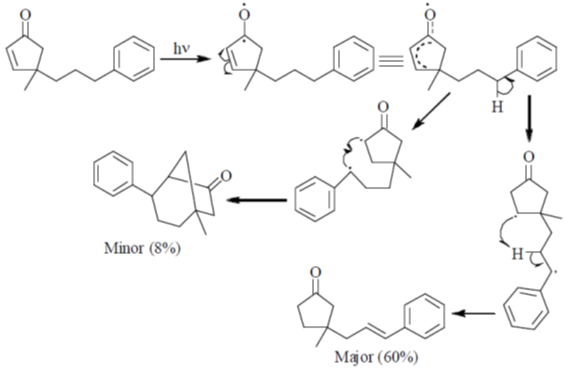 Correct answer is (c)
Correct answer is (c)
91. Correct sequence of reagents to be used for the following conversion is
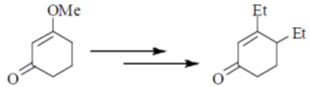
(a) (I) LDA, EtBr; (II) EtLi; (III) H3O+
(b) (I) EtLi, (II) LDA, EtBr; (III) H3O+
(c) (I) H3O+; (II) EtLi; (III) LDA, EtBr
(d) (I) EtLi; (II) H3O+; (III) LDA, EtBr
Ans. a
Sol. 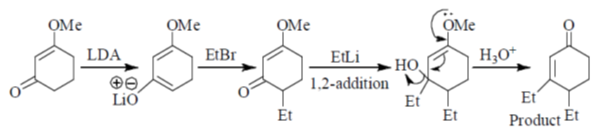
Correct answer is (a)
92. Both, 1-chloro-1-phenylpropan-2-one and 1-chloro-3-phenylpropan-2-one give sam product (A) when heated in presence of NaOMe. The product (A) is
(a) methyl 3-phenylpropanoate
(b) methyl 2-phenylpropanoate
(c) methyl 2-methoxy-2-phenylacetate
(d) 1-methoxy-3-phenylpropan-2-one
Ans. a
Sol. 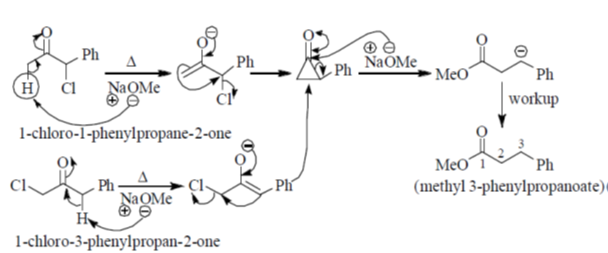
Correct answer is (a)
93. The major product formed in the following reaction sequence is
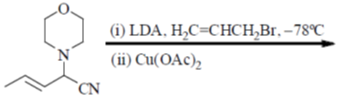
(a) 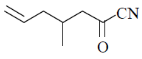
(b) 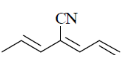
(c) 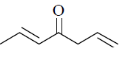
(d) 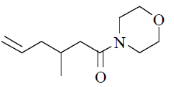
Ans. c
Sol. 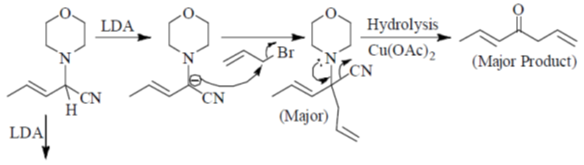
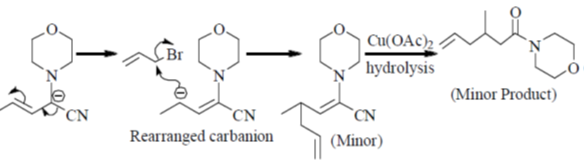
Hydrolysis in presence of Cu(II), salt helps to remove 
Correct answer is (c)
94. The major product formed in the following reaction is
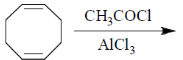
(a) 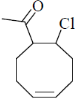
(b) 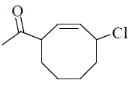
(c) 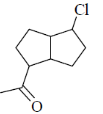
(d) 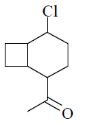
Ans. c
Sol. 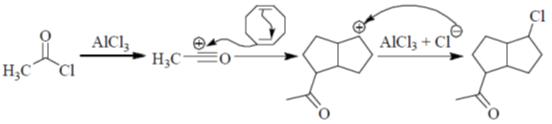
Correct answer is (c)
95. The structures of products (A) and (B) formed in the Edman degradation of the dipeptide are
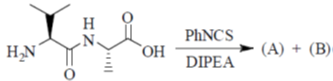
(a) 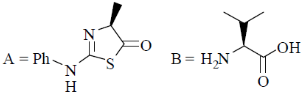
(b) 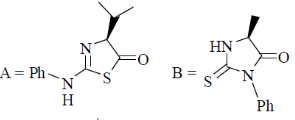
(c) 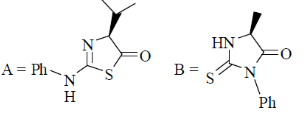
(d) 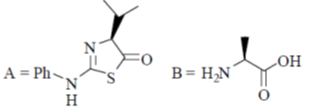
Ans. d
Sol. Edman degradation method is used for N-terminal analysis of protein (Polypeptide). Since, this method can label and cleave the peptide from N-terminal and forms corresponding phenylthiohydonation derivative of amino acid.
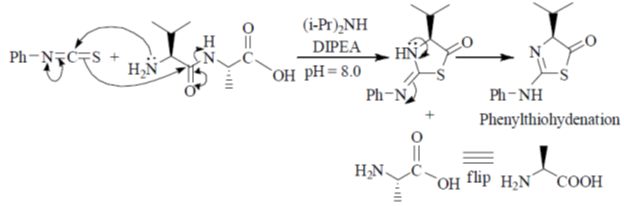
Correct answer is (d)
96. Partial spectroscopic data is given below for an organic compound
(I) 4 signals between 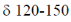 ppm in 13C NMR spectrum
ppm in 13C NMR spectrum
(II) 2 doublets betwen 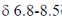 ppm in 1H NMR spectrum
ppm in 1H NMR spectrum
(III) an absorption band at 1724 cm–1 in IR spectrum
The structure of the compound is
(a) 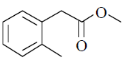
(b) 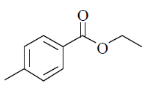
(c) 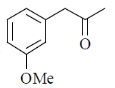
(d) 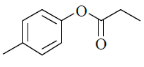
Ans. b
Sol. IR absorption at 1724 cm–1 indicates the conjugated ester.
13C NMR and 1H NMR data shows the 1, 4-disubstitution.
IR = 1724 cm–1 due to resonance effect of aromatic ring.
Correct answer is (b)
97. The major product formed in the following reaction is
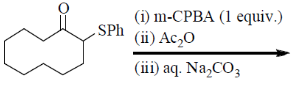
(a) 
(b) 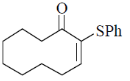
(c) 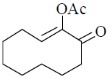
(d) 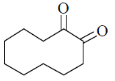
Ans. d
Sol. Pummerer Rearrangement:
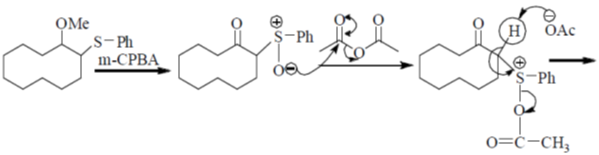
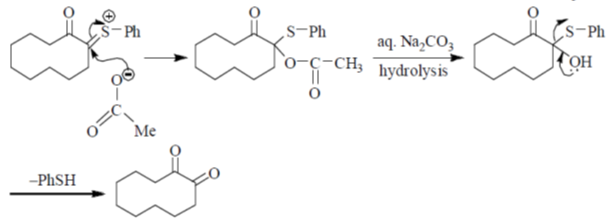
Correct answer is (d)
98. The major products (A) and (B) in the following reaction sequence are
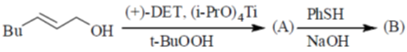
(a) 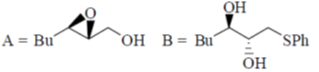
(b) 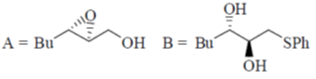
(c) 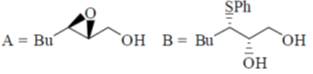
(d) 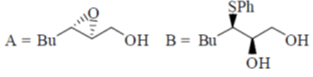
Ans. b
Sol. Payne rearrangement after asymetric epoxidation:
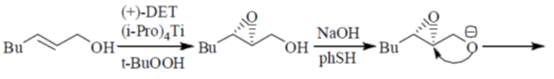
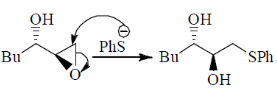
Correct answer is (b)
99. The compound P undergoes a pericyclic reaction under photochemical conditions to give compound Q. In compound Q, the relative stereochemistry and 1H NMR chemical shift values of methyl groups 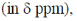 respectively, are
respectively, are
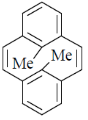
(a) cis; –5
(b) trans; 17
(c) cis; 17
(d) trans; –5
Ans. d
Sol. 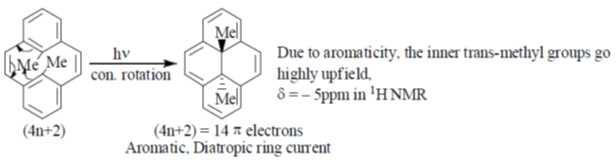
Correct answer is (d)
100. The major products A and B in the following raction sequence are
(a) 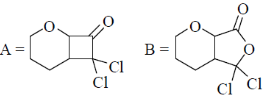
(b) 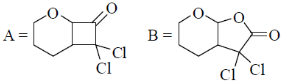
(c) 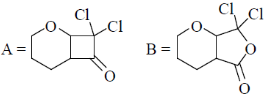
(d) 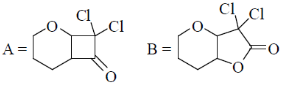
Ans. d
Sol. 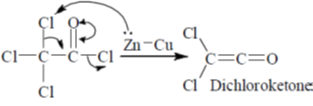
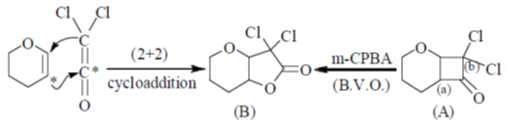
Carbon (a) migrates, not carbon (b) because of two electron withdrawing Cl atoms which cannot stabilize the positive charge in TS.
Correct option is (d)
101. Arrange the following molecules in order of increasing fundamental vibrational frequencies
(a) 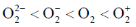
(b) 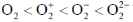
(c) 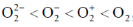
(d) 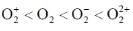
Ans. a
Sol. As bond order increases, stretching frequency increases
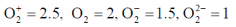
Correct answer is (a)
102. One of the Huckel molecular orbitals of 1, 3-butadiene is
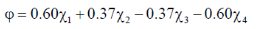
The energy of this orbital in terms of the coulomb 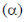 and resonance
and resonance 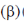 integrals is
integrals is
(a) 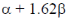
(b) 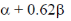
(c) 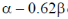
(d) 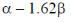
Ans. b
Sol. Given wave function corresponds to one node.
Correct answer is (b)
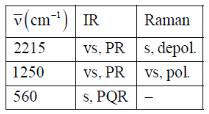
103. A molecule AB2 shows the following IR and Raman spectra
The structure of the molecule is
(a) Linear symmetrical 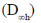
(b) Bent symmetrical (C2v)
(c) Linear asymmetrical 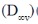
(d) Bent asymmetrical (Cs)
Ans. c
Sol. Since, 2PR and 1PQR band  Linear molecule
Linear molecule
Since, two of the vibrations are active in both IR and RAMAN  assymetric.
assymetric.
Hence, linear assymetric 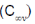
Correct answer is (c)
104. For a one-dimensional (x) harmonic oscillator perturbed by an x3 potential, the sum of the first order and second order corrections to the ground state energy is
(a) < 0
(b) 0
(c) 0
(d) > 0
Ans. a
Sol. For 1-D harmonic oscillator, wavefunction of the particle in the ground state, will have even parity (defined parity). Therefore, first order correction to ground state energy due to a perturbing potential x3 (odd function) will be equal to zero.
For any potential system, the second order correction to ground state energy due to perturbation is always negative.
So, sum of the corrections will be < 0
Correct answer is (a)
105. Difference of average values of position <x> for states n = 1 and n = 2 of a particle confined in a one-dimensional (x) box of length L is
(a) L/4
(b) L/2
(c) L/3
(d) 0
Ans. d
Sol. For a 1-D box of length 'L', the average value of kposition <x> will be same for all states.
So, different in <x> for n = 1 and n = 2 states, will be zero.
Correct answer is (d)
106. The hermitian operator among the following is
(a) 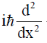
(b) 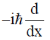
(c) 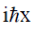
(d) 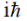
Ans. b
Sol. 
Correct answer is (b)
107. The translational partition function for Ar confined to a volume of 1 L at 300K, having thermal wavelength of 1.60 × 10–11 m, is closest to
(a) 24.4 × 1029
(b) 2.44 × 1029
(c) 0.244 × 1029
(d) 244 × 1029
Ans. b
Sol. 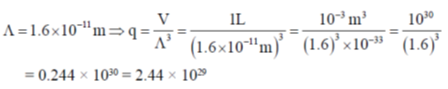
Correct answer is (b)
108. Consider a phase transition between two incompressible phases. the correct statement among the following is
(a) The transition is independent of pressure
(b) The transition is independent of temperature
(c) The entropy of such transition is always zero
(d) The enthalpy of such transition is always non-zero
Ans. a
Sol. Since, the given phases are in comprssible. Therefore, the effect of pressure is zero on this phase transistor.
Correct answer is (a)
109. The third and fourth lines in the rotational Raman spectrum of CO are separated by 8 cm–1. The CO bond length is given by
(a) 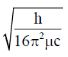
(b) 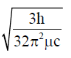
(c) 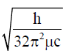
(d) 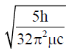
Ans. a
Sol. 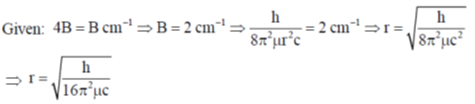
Correct answer is (a)
110. Conductivities of water and a saturated solution of a sparingly soluble salt AB2 are 7 and 21 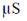 m–1, respectively. Given,
m–1, respectively. Given, 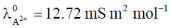 and
and 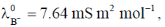 the solubility of AB2, in mol m–3, is
the solubility of AB2, in mol m–3, is
(a) 5.0 × 10–4
(b) 5.0 × 10–3
(c) 5.0 × 10–5
(d) 5.0 × 10–6
Ans. a
Sol. 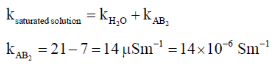
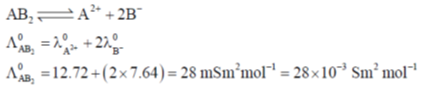
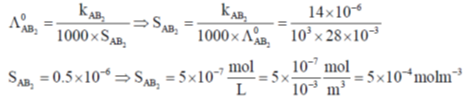
Correct answer is (a)
111. The equilibrium constant of the following reaction:
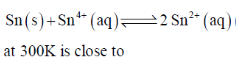
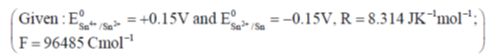
(a) 106.08
(b) 108.08
(c) 1010.08
(d) 1012.08
Ans. c
Sol. 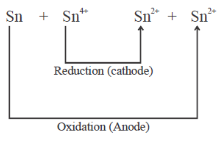
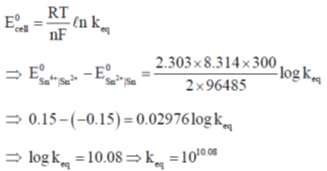
Correct answer is (c)
112. Langmuir adsorption isotherm for the dissociative adsorption of D2(p = partial pressure of D2 and k = ratio of rate constants for adsorption and desorption) is
(a) 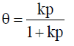
(b) 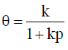
(c) 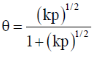
(d) 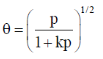
Ans. c
Sol. 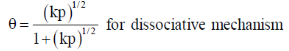
Correct answer is (c)
113. Entropy of a perfect gas is
(a) independent of V
(b) proportional to V
(c) proportional to ln V
(d) proportional to V2
Ans. c
Sol. 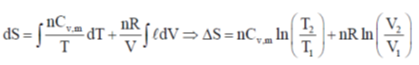
Therefore, entropy is proportional to 
Correct answer is (c)
114. The contour and root mean square length (in nm) of a polymer chain modelled as a random coil, with N = 1000 and l = 150 pm, are closest to
(a) 1.50 and 47.4
(b) 15.0 and 4.74
(c) 150 and 47.4
(d) 150 and 4.74
Ans. d
Sol. Contour 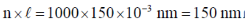
And root mean square length 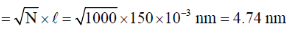
Correct answer is (d)
115. The free energy [A – A(0)] of a system with 10 non-interacting spins (S = 1) is
(a) 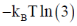
(b) 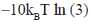
(c) 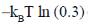
(d) 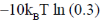
Ans. b
Sol. 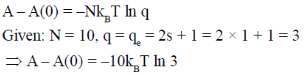
Correct answer is (b)
Table-1:
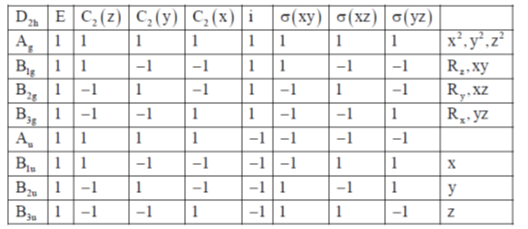
116. The 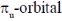 of ethylene, when placed in the xy plane with the C = C bond aligned to the x-axis transforms according to the irreducible representation (Use Table-1)
of ethylene, when placed in the xy plane with the C = C bond aligned to the x-axis transforms according to the irreducible representation (Use Table-1)
(a) au
(b) b1u
(c) b2u
(d) b3u
Ans. b
Sol. If ethylene molecule is in xy plane (As in english version)
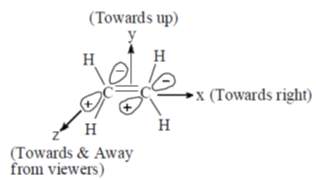
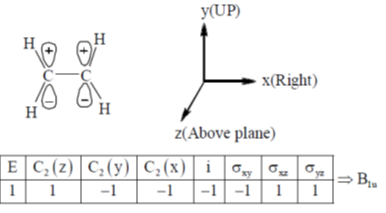
117. The 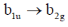 transition in ethylene is
transition in ethylene is
(a) not allowed
(b) allowed by to x-polarized light
(c) allowed by y-polarized light
(d) allowed by z-polarized light
(Use Table-1)
Ans. d
Sol. 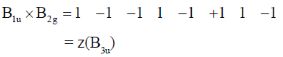
Correct answer is (d)
118. A metal crystallizes with cubic close-packed structure. The 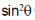 values of Bragg reflections of Miller Planes (200) and (111) are 0.18 and 0.14, respectively. The unit cell length is
values of Bragg reflections of Miller Planes (200) and (111) are 0.18 and 0.14, respectively. The unit cell length is
(a) 
(b) 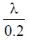
(c) 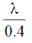
(d) 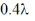
Ans. c
Sol. 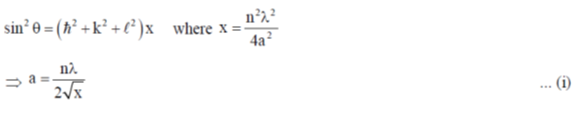
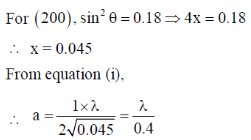
Correct answer is (c)
119. kuni is the effective first-order rate constant of the following unimolecular reaction
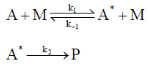
The slope and intercept of the plot of 1/kuni vs. 1/[M] are 4 × 106 and 8 × 1011, respectively. The value of k–1/k2 is
(a) 2 × 105
(b) 0.5 × 105
(c) 32 × 105
(d) 2 × 10–5
Ans. a
Sol. 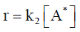 ... (i)
... (i)
Applying Steady state approximation on A*
Rate of formation of A* = rate of deformation of A*
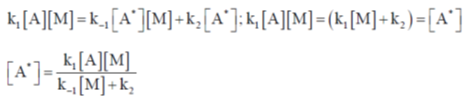
On substituting the value of [A*] in equation (i)
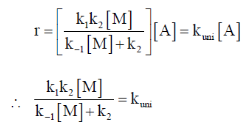
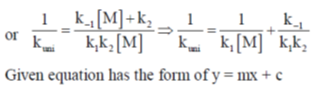

Correct option is (a)
120. The decomposition mechanism of ozone is
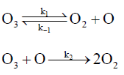
If 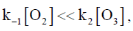 then the order of the reaction with respect to ozone is
then the order of the reaction with respect to ozone is
(a) zero
(b) one
(c) two
(d) complex
Ans. b
Sol. r = k2[O3][O] ... (i)
Applying SSA on O gives
Rate of consumption of [O] = Rate of formation of [O]
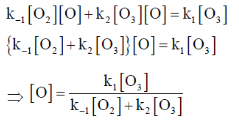
On substituting the value of [O] in equation (i)
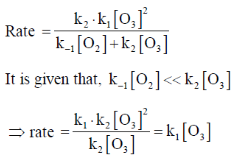
Hence, order with respect to ozone is 1.
Correct option is (b)
