CSIR NET CHEMISTRY (DEC-2013)
Previous Year Question Paper with Solution.
21. L – DOPA is used for the treatment of
(a) Tuberculosis
(b) Parkinson's disease
(c) Diabetes
(d) Cancer
Ans. b
Sol. L – DOPA is used for Parkinsons' disease.
Correct answer is (b)
22. In the IR spectrum of p – nitrophenyl acetate, the carbonyl absorption band appears at
(a) 1660 cm–1
(b) 1700–1
(c) 1730 cm–1
(d) 1770 cm–1
Ans. d
Sol. 
p – nitrophenoxy group will be strong electron withdrawing group for carbonyl so, C = O stretching frequency will increase from normal ester to 1770 cm–1.
Correct answer is (d)
23. The major product formed in the reaction of styrene with an excess of lithium in liquid ammonia and t – butyl alcohol is:
(a) 
(b) 
(c) 
(d) 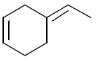
Ans. d
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct option is (d)
24. The major product formed in the following reaction is

(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. Chemical reaction involved in the above transformation can be illustrated as


Correct option is (c)
25. For estrone, among the statements A–C, the correct ones are
A. It is a steroidal hormone
B. It has two hydroxyl groups
C. It has one ketone and one hydroxyl groups
(a) A, B and C
(b) A and B
(c) A and C
(d) B and C
Ans. c
Sol. The structure of estrone is

Correct answer is (c)
26. An organic compound having the molecular formula C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR spectrum. The compound is
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. 
1H NMR : Singlet 12H, singlet 2 H
13C NMR : CH3 Signal, CH signal, C signal.
Correct answer is (a)
27. Amongst the following, the compound which has the lowest energy barrier for the cis – trans isomerisation is:
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. 
Among all options, option (c) has gain aromaticity in both the rings. Therefore, the lowest energy barries for cis – trans isomerisation is option (c)
28. The IUPAC name of the compound given below is

(a) (2E, 4E) – 3 – chlorohexa – 2, 4 – diene – 1, 6 – diol
(b) (2Z, 4E) – 3 – chlorohexa – 2, 4 – diene – 1, 6 – diol
(c) (2E, 4Z) – 3 – chlorohexa – 2, 4 – diene – 1, 6 – diol
(d) 4(2E, 4Z) – 3 – chlorohexa – 2, 4 – diene – 1, 6 – diol
Ans. b
Sol. 
Correct answer is (b)
29. The major product formed in the following reaction is

(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct option is (c)
30. The constituent amino acids present in the following dipeptide, respectively, are

(a) (R) – aspartic acid and (S) – lysine
(b) (S) – aspartic acid and (R) – lysine
(c) (R) – glutamic acid and (S) – arginine
(d) (S) – glutamic and (S) – arginine
Ans. a
Sol. Constituent amino acid present in above dipeptide can be shown as


Correct option is (a)
31. A suitable organocatalyst for enantioselective synthesis of Wieland – Miescher ketone (A) is

(a) (–) – proline
(b) (+) – menthone
(c) guanidine
(d) (+) – BINOL
Ans. a
Sol. This reaction is an example of Robinson annulation. In this reaction we required a base and for the synthesis of optically active compound (A) we will have to use chiral base among all the option only protein is naturally occuring base.

Correct answer is (a)
32. For acylation with acetic anhydride / trienthylamine and oxidation with chromium trioxide of the transand cis – alcohols A and B, the correct statement is
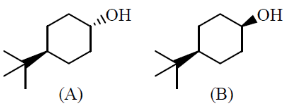
(a) A undergoes acylation as well as oxidation faster than B
(b) B undergoes acylation as well as oxidation faster than A
(c) A undergoes acylation faster than B, whereas B undergoes oxidation faster than A
(d) B undergoes acylation faster than A, whereas A undergoes oxidation faster than B
Ans. c
Sol. 


'A' undergoes acylation faster while as 'B' undergoes oxidation faster.
Correct answer is (c)
33. The two benzylic hydrogens HA and HB in the compounds I and II, are

(a) diastereotopic in I and enantiotopic in II
(b) diastereotopic in II and enantiotopic in I
(c) diastereotopic in both I and II
(d) diastereotopic in both I and II
Ans. b
Sol. 
Correct answer is (b)
34. The following reaction proceeds through a

(a) 1, 3 – sigmatropic rearrangement
(b) 2, 3 – sigmatropic rearrangement
(c) 3, 3 – sigmatropic rearrangement
(d) 3, 5 – sigmatropic rearrangement
Ans. c
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct option is (c)
35. The number of nodes present in the highest occupied molecular orbital of 1, 3, 5 – hexatriene in its ground state is
(a) One
(b) Two
(c) Three
(d) Four
Ans. b
Sol. 

[Here nodes are represented by dot (•)]
There are two node present in the highest occupied molecular orbital HOMO of 1, 3, 5 hexatrine.
Correct option is (b)
36. Deuterium kinetic isotope effect for the following reaction was found to be 4.0. Based on this information, mechanism of the reaction is

(a) E1
(b) E2
(c) E1CB
(d) free radical
Ans. b
Sol. Kinetic isotope effect is seen in slow step. In case of E2 – reaction, 'H' or 'D' is involved.
Correct answer is (b)
37. The major product formed in the following reaction is

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Correct answer is (b)
38. The bond order of the metal - metal bond in the dimeric complex  is
is
(a) 4.0
(b) 3.5
(c) 3.0
(d) 2.5
Ans. b
Sol. 

Correct option is (b)
39. The reaction on FeCl3.6H2O with SOCl2 yields.
(a) FeCl2(s), SO2(g) and HCl (g)
(b) FeCl3(s), SO2(g) and HCl (l)
(c) FeCl2(s), SO3(g) and HCl (g)
(d) FeCl3(s), SO2(g) and HCl (g)
Ans. d
Sol. 
Correct answer is (d)
40. Patients suffering from Wilson's disease have
(a) Low level of Cu – Zn superoxide dismutase
(b) High level of Cu – Zn superoxide dismutase
(c) Low level of cooper – storage protein, ceruloplasmin
(d) High level of cooper – storage protein, ceruloplasmin
Ans. c
Sol. Wilson's disease is caused by excess accumulation of Cu in the body.
Correct answer is (c)
41. High does of dietary supplement ZnSO4 for the cure of Zn deficiency
(a) reduces myoglobin
(b) increases iron level in blood
(c) increases cooper level in brain
(d) reduces copper, iron and calcium levels in body
Ans. d
Sol. Correct option is (d)
42. Which of the following in NOT suitable as catalyst for hydroformylation?
(a) HCo(CO)4
(b) HCo(CO3)PBu3
(c) HRh(CO(PPh3)3
(d) H2Rh(PPh3)2Cl
Ans. d
Sol.  and
and  These all are the catalyst for hydroformylation process.
These all are the catalyst for hydroformylation process.
Correct answer is (d)
43. Commonly used scintillator for measuring radiation is
(a) NaI(AI)
(b) Nal(TI)
(c) CsI (TI)
(d) CsI(AI)
Ans. b
Sol. Correct answer is (b)
44. A sample of aluminium ore (having) no other metal) is dissolved in 50 mL of 0.05 M EDTA. for the titration of unreacted EDTA, 4 mL of 0.05 M MgSO4 is required. The percentage of Al in the sample is:
(a) 27
(b) 31
(c) 35
(d) 40
Ans. b
Sol. Correct answer is (b)
45. In a cluster, H3CoRu3(CO)12, total number of electrons considered to be involved in its formation is
(a) 57
(b) 60
(c) 63
(d) 72
Ans. b
Sol. 
3 + 9 + 8 × 3 + 12 × 2 = 60 electron.
Correct answer is (b)
46. Among the following, the correct acid strength thrend is represented by
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. 
Reference – Shriver Atkin (P. No. 123  Bronsted acid base concept)
Bronsted acid base concept)
Correct answer is (c)
47. Among the molten alkali metals, the example of an immiscible pair (in all proportions) is
(a) K and Na
(b) K and Cs
(c) Li and Cs
(d) Rb and Cs
Ans. c
Sol. Large difference between the size of Li and Cs. So, it is difficult to get a solid solution of these two metals.
Correct answer is (c)
48. Among the following, an example of a hypervalent species is
(a) BF3.OEt2
(b) SF4
(c) [PF6]–
(d) Sb2S3
Ans. c
Sol. More than 8 electrons in the valence shell of P in 
Correct answer is (c)
49. An octahedral metal ion M2+ has magnetic moment of 4.0 B.M. The correct combination of metal ion and d-electron configuration is given by
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. 

Correct answer is (a)
50. According to VSEPR theory, the geometry (with lone pair) around the central iodine in I3+ and I3– ions respectively are
(a) tetrahedral and tetrahedral
(b) trigonal bipyramidal and trigonal bipyramidal
(c) tetrahedral and trigonal bipyramidal
(d) tetrahedral and octahedral
Ans. c
Sol. Number of valence electrons in  central atom are 8 while in
central atom are 8 while in  are 10 and hence the geometries are tetrahedral and trigonal bipyramidal.
are 10 and hence the geometries are tetrahedral and trigonal bipyramidal.
Correct answer is (c)
51. Treatment of CIF3 with SbF5 leads to the formation of a/an
(a) polymeric material
(b) covalent cluster
(c) ionic compound
(d) lewis acid-base addcut
Ans. c
Sol. 
SbF5 is a very strong F – acceptro and leads to the formation of ionic compound.
Correct answer is (c)
52. The reason for the chemical inertness of gaseous nitrogen at room temperature1 is best given by its
(a) high bonding energy only
(b) electronic configuration
(c) HOMO–LUMO gap only
(d) high bond energy and HOMO–LUMO gap
Ans. d
Sol. 
High bond energy and HOMO – LUMO gap.
Correct answer is (d)
53. Two tautomeric forms of phosphorus acid are
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. H3PO3 is a dibasic and reducing in nature.
Correct answer is (a)
54. The correct thermodynamics relation among the following is
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. 

Correct option is (a)
55. The boiling point of a solution of non-volatile solid is higher than that of the pure solvent, It always indicates that
(a) the enthalpy of the solution is higher than that of the pure solvent.
(b) the entropy of the solution is higher than that of the pure solvent.
(c) the Gibbs free energy of the solution is higher than that of the pure solvent.
(d) the internal energy of the solution is higher than that of pure solvent.
Ans. b
Sol. Correct answer is (b)
56. According to Arrhenius equation (K = rate constant and T = temperature)
(a) In K decreases lineraly with 1/T
(b) In K decreases lineraly with T
(c) In K increases lineraly with 1/T
(d) In K increases lineraly with T
Ans. a
Sol. 
Hence, ln K decreases lineraly as l/T increases.
Correct answer is (a)
57. The angle at which the first order Bragg reflection is observed from (110) plane in a simple cubic unit cell of side 3.238Å, when chromium  radiation of wavelength 2.29Å is used, is
radiation of wavelength 2.29Å is used, is
(a) 300
(b) 450
(c) 600
(d) 900
Ans. a
Sol. 
Correct answer is (a)
58. The orbital with two radiaal and two angular nodes is
(a) 3p
(b) 5d
(c) 5f
(d) 8d
Ans. b
Sol. For radial node = n – 1 – 1 = 5 – 2 – 1 = 2
Angular node = l = 2
Correct answer is (b)
59. Michael Faraday observed that the colour of colloidal suspensions of gold nanoparticles changes with the size of the nanoparticles. This is because
(a) Gold forms complex with the solvent.
(b) Band gap of gold changes with size of the nanoparticle.
(c) Gold in nanocrystalline form undergoes transmutation to other elements.
(d) Colloidal suspensions diffract light
Ans. b
Sol. Correct answer is (b)
60. The energy 2s and 2p orbitals is the same for
(a) Li
(b) Li2+
(c) Be2+
(d) H–
Ans. b
Sol. 
Li has one electron in 2s and no electron in 2p hence the energies of 2s and 2p orbitals will be different.
Li++ has only one electron in ls1 hence will have no screening effect therefore, 2s and 2p will have same energies.
Correct answer is (b)
61. If a homonuclear diatomic molecule is oriented along the Z-axis, the molecular orbital formed by linear combination of p, orbitals of the two atoms is
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. If a homonuclear diatomic molecule is oriented the z – axis, then linear combination of pz orbitals of two atoms form  – orbitals.
– orbitals.
The molecular orbitals formed by linear combination of px or py orbitals of two atoms is 
Correct answer is (c)
62. A reaction contains a mixture of N2, H2 and NH3 in equilibrium (Kp = 3.75 atm–2). If sufficient. He is introduced into the reactor to double the total pressure, the value of KP at the new equilibrium would be
(a) 0.94 atm–2
(b) 3.75 atm–2
(c) 7.50 atm–2
(d) 15.00 atm–2
Ans. b
Sol. kp changes only by change in temperature.
Correct answer is (b)
63. The volume of a gas absorbed on a solid surface is 10.0 ml, 11.0 ml, 12.2 ml, 14.5 ml and 22.5 ml at 1.0, 2.0, 3.0, 4.0 and 5.0 atm, pressure, respectively. These data are best represented by
(a) Gibb's isotherm
(b) Langmuir isotherm
(c) Freundilich isotherm
(d) BET isotherm
Ans. d
Sol. When we plot  graph it increasing gradually and multilayered is formed. No monolayer is cormed.
graph it increasing gradually and multilayered is formed. No monolayer is cormed.
Correct option is (d)
64. A compound of M and X atoms has a cubic unit cell. M atoms are at the corners and body centre position and X atoms are at face centre positions of the cube. The molecular formula of the compound is
(a) MX
(b) MX2
(c) M3X2
(d) M2X3
Ans. d
Sol. 
Correct answer is (d)
65. When Frenkel defects are created in an otherwise perfect ionic crystal, the density of the ionic crystal
(a) increases
(b) decreases
(c) remains same
(d) oscillates with the number of defects
Ans. c
Sol. In case of Frenkel defect, ions are not missing from the lattice. They just displace into interstitial sites. Also, no extra ion come from outside into the lattice. Therefore, density reamisn same.
Correct answer is (c)
66. The molecule in which the bond order increases upon addition of an electron is
(a) O2
(b) B2
(c) P2
(d) N2
Ans. b
Sol. 
If an electron is added to B2, then will go bonding to  – orbital.
– orbital.
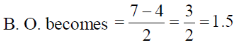
Correct answer is (b)
67. In a potentiometric titration, the end point is obtained by observing
(a) change in colour
(b) jump in potential
(c) increase in current
(d) increase in turbidity
Ans. b
Sol. Correct answer is (b)
68. Electrolysis of an aqueous solution of 1.0 M NaOH results in
(a) Na at the cathode and O2 at the anode
(b) H2 at the cathode and O2 at the anode
(c) Na and H2 at the cathode and O2 at the anode
(d) O2 at the cathode and H2 at the anode.
Ans. b
Sol. 
Correct answer is (b)
69. The cell voltage of Daniel cell  is 1.07 V. If reduced potential of
is 1.07 V. If reduced potential of  is 0.34 V, the reduction potential of
is 0.34 V, the reduction potential of  is
is
(a) 1.141 V
(b) – 1.41 V
(c) 0.73 V
(d) –0.73 V
Ans. d
Sol. 
Correct answer is (d)
70. In the mechanism of reaction, H2+Br2 2HBr, the first stepis
2HBr, the first stepis
(a) dissociation of H2 into H• radicals
(b) dissociation of Br2 into Br• radicals
(c) reaction of H• radical with Br2
(d) reaction of Br• radical with H2
Ans. b
Sol. In this reaction, the first step is chain initiation step, in which the bromine molecule acquires energy as a result of collision with another bromine molecule to dissociate into two Br atoms.
Correct answer is (b)
71. For an electronic configuration of two non-equivalent  electronics
electronics  which of the following terms is not possible?
which of the following terms is not possible?
(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. For two non – equivalent 

Correct option is (d)
72. Consider a two – dimensional harmonic oscillator with potential energy  If
If  are the eigensolutions and
are the eigensolutions and  are the eigenvalues of harmonic oscillator problem in x and y direction with potential
are the eigenvalues of harmonic oscillator problem in x and y direction with potential  respectively, the wave function and eigenvalues of the above two – dimensional harmonic oscillator problem are
respectively, the wave function and eigenvalues of the above two – dimensional harmonic oscillator problem are
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. 


Correct option is (c)
73. The quantum mechanical virial theorem for a general potential  is given by
is given by  where T is the kinetic energy operator and < > indicates expectation value. This leads to the following relation between the expectation value of kinetic energy and potential energy for a quantum mechanical harmonic oscillator problem with potential
where T is the kinetic energy operator and < > indicates expectation value. This leads to the following relation between the expectation value of kinetic energy and potential energy for a quantum mechanical harmonic oscillator problem with potential 
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. 

Correct option is (a)
74. Consider a particle in a one dimensional box of length 'a' with the following potential

Starting with the standard particle in a box hamiltonian as the zeroth order Hamiltonian and the potential of V1 from 'a/2' to 'a' as a perturbation, the first – order energy correction to the ground state is
(a) V1
(b) V1/4
(c) –1V1
(d) V1/2
Ans. d
Sol. Particle in a box hamiltonian as zeroth order hamiltonian and potential vi from a/2 to a.



Correct option is (d)
75. The most porbable value of 'r' for an electron in 1s orbital of hydrogen atom is
(a) a0/2
(b) a0
(c) 
(d) 
Ans. b
Sol. 


Correct option is (b)
76. The angular momentum operator  is
is
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. 


Correct option is (b)
77. The molecule with the smallest rotation partition function at any temperature among the following is
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Since rotational partition function is given by

Hence, two factor should be considered

In present case,  has smallest
has smallest  (reduced mass) also having
(reduced mass) also having 
Therefore,  will have lowest rotational partition function.
will have lowest rotational partition function.
Correct option is (b)
78. Both NaCl and KCl crystallize with the FCC structure. However, the X-ray powder diffraction pattern of NaCl corresponds to be FCC structure whereas, that of KCI corresponds to simple cubic structure. This is because
(a) K+ and Cl– are isoelectronic
(b) Na+ and Cl– are isoelectronic
(c) K+ and Cl– are disordered in the crystal lattice
(d) KCl has anti – site defects.
Ans. a
Sol. Since, K+ and Cl– are isoelectric, some of the peaks are missing entirely. The scattering intensities for X – rays are directly related to the number of electrons in the atom. Light atoms scatter X – rays weakly, while heavy atoms scatter X – rays more effectively.
Correct answer is (a)
79. Consider the cell:

Ecell = 1.71V at 250C for the above cell. The equilibrium constant for the reaction:
 at 250C would be close to
at 250C would be close to
(a) 1027
(b) 1054
(c) 1081
(d) 1040
Ans. b
Sol. 

Correct option is (b)
80. The molecule that has the smallest diffusion coefficient in water is
(a) glucose
(b) fructose
(c) ribose
(d) surcrose
Ans. d
Sol. Diffusion coefficient D

which measures the mobility of the ion due to its thermal energy.
Diffusion is the process which involves the migration of a component in solution down a gradient of itw own concentration.
Diffusion coefficient in water of sucrose is smallest because its mobility is very low. It contains high concentration of ion. so, migration of component in solution very small.
Correct answer is (d)
81. Metallic gold crystallizes in FCC structure with unit cell dimension of 4.00 Å. The atomic radius of gold is
(a) 0.866Å
(b) 1.414Å
(c) 1.732Å
(d) 2.000Å
Ans. b
Sol. We know that, for FCC atomic radius is given by

Correct answer is (b)
82. A first order gaseous reaction is 25% complete in 30 minutes at 2270C and in 10 minutes at 2370C. The activation energy of the reaction is closest to (R = 2 cal K–1 mol–1)
(a) 27 kcal mol–1
(b) 110 kcal mol–1
(c) 55 kcal mol–1
(d) 5.5 kcal mol–1
Ans. c
Sol. From Arrhenius, equation, we have

Correct answer is (c)
83. In the reaction between NO and H2 the following data are obtained

The orders with respect to H2 and NO are
(a) 1 with respect to NO and 2 with respect to H2
(b) 2 with respect to NO and 1 with respect to H2
(c) 1 with respect to NO and 3 with respect to H2
(d) 2 with respect to NO and 2 with respect to H2
Ans. b
Sol.  where a is order w.r.t. NO & b is order w.r.t. H2
where a is order w.r.t. NO & b is order w.r.t. H2
From experiment I, on doubling pressure of NO rate becomes 4 times
Therefore, a = 2
From experiment II, on doubling pressure of H2 rate becomes almost double.
Therefore, b = 1

Correct option is (b)
84. The energy for a single electron excitation in cyclopropenium cation in Hückel theory is
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. 



Correct option is (c)
85. The atomic masses of fluorine and hydrogen are 19.0 and 1.0 amu, respectively (1 amu = 1.67×10–27 kg). The bond length of HF is 2.0Å. The moment of inertia of HF is
(a) 3.2×10–47 kg m2
(b) 6.4×10–47 kg m2
(c) 9.6×10–47 kg m2
(d) 4.8×10–47 kg m2
Ans. b
Sol. F atomic mass = 19.0 amu.
H atomic mass = 1 amu
H – F bond length = 2.0 Å

Correct option is (b)
86. The masses recorded when a substance is weighed 4 times are 15.8, 15.4, 15.6 and 16.0 mg. The variance (square of the standard deviation) is closest to
(a) 0.02
(b) 0.05
(c) 0.10
(d) 0.20
Ans. b
Sol. 

Correct option is (b)
87. The transition that is allowed by x-polarized light in trans-butadiene is
(The character table for C2h is given below)

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. If direct product of ground and excited state transforms according to the x, y and z axiz then the transition will be allowed.

and Bu transforms as per x–axis. Hence, Bg to Au transition will be x – polarized.
Correct option is (b)
88. The heat capacity of 10 mol of an ideal gas at a certain temperature in 300 JK–1 at constant pressure. The heat capacity of the same gas at the same temperature and at constant volume would be
(a) 383 JK–1
(b) 217 JK–1
(c) 134 JK–1
(d) 466 JK–1
Ans. b
Sol. 
Correct answer is (b)
89. The Maxwell's relationship derived from the equation dG = VdP – SdT is
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. dG = VdP = SdT ...(i)
In an isobaric – isothermal process
dP = 0 and dT = 0
dG = 0 or G = constant
Thus Gibb's function is that physical quantity which remains constant in reversible isobaric process
From equation (i)
G = G (P, T)

From equation (i) and (ii)

And since dG is a percent differential

Correct option is (c)
90. The chemical potential  of the ith component is defined as
of the ith component is defined as
(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. The chemical potentials are thus the rate of change of free energy per mole at constant temperature and constant pressure.
 and constant temperature pT - 0 and constant pressure dP=0
and constant temperature pT - 0 and constant pressure dP=0

Correct answer is (d)
91. Work (w) involved in isothermal reversible expansion from Vi to Vr of n moles of an ideal gas is
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Work done is reversible expansion process for an ideal gas

If gas expend from volume, Vi to Vf if process is isothermal then we may integrate it from Vi to Vf,

Correct answer is (a)
92. The limiting molar conductivities of NaCl, Nal and RbI are 12.7, 10.8 and 9.1 mS m2mol–1, respectively. The limiting molar conductivity of RbCl would be
(a) 32.6 mS m2 mol–1
(b) 7.2 mS m2 mol–1
(c) 14.4 mS m2 mol–1
(d) 11.0 mS m2 mol–1
Ans. d
Sol. 
Correct answer is (d)
93. The number of ways in which four molecules can be distributed in two different energy levels is
(a) 6
(b) 3
(c) 16
(d) 8
Ans. c
Sol. Number of energy levels (gi) = 2
Number of molecules (Ni) = 4
The number of ways of distribution 
(since, it is not given either particles are distinguishable or indistinguishable, therefore, we will consider it as distinguishable)
Correct option is (c)
94. An element exists in two crystallographic modifications with FCC and BCc structures. the ratio of the densities of the FCC and BCC modifications in terms of the volumes of their unit cells (VFCC and VBBC) is
(a) VBBC : VFCC
(b) 2VBCC : VFCC
(c) VBCC : 2VFCC
(d) 
Ans. b
Sol. 
Correct option is (b)
95. Given  The resonance frequency of a proton in magnetic field of 12.6 T is close
The resonance frequency of a proton in magnetic field of 12.6 T is close 
(a) 60 MHz
(b) 110 MHz
(c) 540 MHz
(d) 780 MHz
Ans. c
Sol. 
Correct answer is (c)
96. In Mössbauer experiment, a source emitting at  had to be move towards absorber at 2.2 mm s–1 for resonance. The shift in the frequency between the source and the absorber is
had to be move towards absorber at 2.2 mm s–1 for resonance. The shift in the frequency between the source and the absorber is
(a) 15.0 MHz
(b) 20. 0 MHz
(c) 25.5 MHz
(d) 30.0 MHz
Ans. c
Sol. 
Correct answer is (c)
97. Among the following, the correct combination of complex and its color is
(a) Complex :  Color : Red
Color : Red
(b) Complex :  Color : Orange
Color : Orange
(c) Complex :  Color : Blue
Color : Blue
(d) Complex :  Color : Yellow
Color : Yellow
Ans. c
Sol. Order of ligand is spectro chemical series

From above it is clear that CN– will produce highest energy difference, followed by F–, Cl–, SCN–. As colour of complex is complementary colour of absorbed light.
Thus  absorbs orange colour radiation which complementary colour is blue therefore colour of
absorbs orange colour radiation which complementary colour is blue therefore colour of  is blue.
is blue.
The other combination according to energy are mismatched. Hence, only option (c) is correct.
Correct answer is (c)
98. In a specific reaction, hexachlorocyclotriphosphazene, N3P3Cl6 was reacted with a metal fluoride to obtain mixed halo derivatives namely N3P3ClF(A), N3P3Cl4F2(B), N3P3Cl2F4(D), N3P3CIF5(E). Compositions among these which can give isomeric products are
(a) A, B and C
(b) B, C and D
(c) C, D and E
(d) E, A and B
Ans. b
Sol. (A) N3P3Cl5F = only one isomer





Correct option is (b)
99. Xenon forms several fluorides and oxofluorides which exihibit acidic behaviour. The correct sequence of descending Lewis acidity among the given species is represented by
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Relative acidic strength of xenon fluorides follows order
XeF6 > XeO2F4 > XeO4 > XeOF4 > XeF4 > XeO2F2 > XeO3
This order depends upon
(i) number of lone pair (ii) number of 'F' atoms
Correct answer is (a)
100. Number of isomeric derivaties possible for the neutral closo – carborane, C2B10H12 is
(a) three
(b) two
(c) four
(d) six
Ans. a
Sol. 
C2B10H12 is show three isomeric derivative which is depend on the temperature.
Correct answer is (a)
101. For higher boranes 3c – 2e 'BBB' bond may be a part of their structures. In B5H9, the number of such electron deficient bond(s) present is/are
(a) four
(b) two
(c) zero
(d) one
Ans. d
Sol. 

Correct option is (d)
102. In the atomic absorption spectroscopic estimation of Fe(III) using O2/H2 flame, the absorbance decreases with the addition of
(a) 
(b) 
(c) EDTA
(d) Cl–
Ans. b
Sol. Chemical reactions between analyte and matrix that prevent the atomization of the analyte. One common one is that Sulfate or phosphates anions will form non – volatile salts with Fe+3, so its signal decreases. Can be treated in two ways 1. Releasing agents – can add to metal to prevent formation of interference complexes. this this example EDTA or 8 – hydroxyquino line.
Correct option is (b)
103. In a polarographic estimation, the limiting currents  were 0.15, 4.65, 9.15 and 27.15 when concentration (mM) of Pb(II) were 0, 0.5 1.0 and 3.0 respectively. An unknown solution of Pb(II) gives a limiting current of
were 0.15, 4.65, 9.15 and 27.15 when concentration (mM) of Pb(II) were 0, 0.5 1.0 and 3.0 respectively. An unknown solution of Pb(II) gives a limiting current of  Concentration of Pb(II) in the unknown is
Concentration of Pb(II) in the unknown is
(a) 1.355 mM
(b) 1.408 mM
(c) 1.468 mM
(d) 1.500 mM
Ans. d
Sol. Limiting current (id)

Limiting current proportional to concentration


Correct option is (d)
104. The gases SO2 and SO3 were reacted separately with CIF gas under ambient conditions. The major products expected from the two reactions respectively, are
(a) SOF2 and CIOSO2F
(b) SOF2 and SO2F2
(c) SO2CIF and SO2F2
(d) SO2CIF and CIOSO2F
Ans. d
Sol. 
Correct answer is (d)
105. The correct statement regarding terminal/bridging CO groups in solid Co4(CO)12 and Ir4(CO)12 is
(a) both have equal number of bridging CO groups
(b) number of bridging CO groups in Co4(CO)12 is 4
(c) the number of terminal CO groups in Co4(CO)12 is 8
(d) the number of bridging CO groups in Ir4(CO)12 is zero
Ans. d
Sol. 
Correct answer is (d)
106. On reducing Fe3(CO)12 with an excess of sodium, a carbonylate ion is formed. The iron is isoelectronic with
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. 
Correct answer is (b)
107. The correct statement for ozone is
(a) It absorbs radiations in wavelength region 290 – 320 nm.
(b) It is mostly destroyed by NO radical in atmosphere
(c) It is non toxic even at 100 ppm level
(d) Its concentration near poles is high due to its paramagnetic nature.
Ans. a
Sol. (i) Ozone is diamagnetic in nature
(ii) It is non toxic even at 1ppm level
(iii) It is not destroyed by no radial in atmosphere
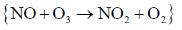
(iv) It absorbs radiations in wave length region 290 – 320 nm
Correct answer is (a)
108. Among the following clusters,

H is encapsulated in
(a) A only
(b) B only
(c) B and C only
(d) A and B only
Ans. a
Sol. 
Correct answer is (a)
109. The solid state structure of aluminum fluoride is
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. A1F3 has octahedral arrangement in 3 – dimethyl structure which causes high methyl point of A1F3 in comparison to A1C13, A1Br3 and AlI3. AlCl3 dimer has layered structure.
Correct answer is (c)
110. Oxidised form of enzyme catalase (structure A); prepared by the reaction of  with H2O2, has green color because
with H2O2, has green color because

(a) Oxidation sate of iron changed from FeIII to FeIV.
(b) Porphyrin ring is oxidized by one electron
(c)  transition appears in the visible region
transition appears in the visible region
(d) FeIV is coordinated with anionic tyrosinate ligand in axial position.
Ans. a
Sol. Catalase is a tetramer of four polypeptide chains covering 50 amino acids.

The colour arises as Fe(III) changes into Fe(IV).
Correct answer is (a)
111. The reactive position of nicotinamide adenine dinucleotide (NAD) in biological redox reactions is
(a) 2 – position of the pyridine ring
(b) 6 – position of the pyridine ring
(c) 4 – position of the pyridine ring
(d) 5 – position of the pyridine ring
Ans. c
Sol. 
Therefore the 4 – position of the pyridine ring is reactive position in the biological reaction.
Correct answer is (c)
112. The electrophiel Ph3C+ reacts with  to give a product A. The product A is formed because
to give a product A. The product A is formed because
(a) Fe is oxidised
(b) Alkyl is substituted with Ph3C
(c) Fe – Ph bond is formed
(d) Alkyl is converted to alkene
Ans. d
Sol. 
5 + 8 + 4 + 1 = 18 electron
Correct answer is (d)
113. Substitution of L with other ligands will be easiest for the species
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. In case of (c) the chances of conversion of haptacity from  is more due to partial bond fixation.
is more due to partial bond fixation.
Correct option is (c)
114. Among the following, the correct statement is
(a) CH is isolobal to Co(CO)3
(b) CH2 is isolobal to Ni(CO)2
(c) CH is isolobal to Fe(CO)4
(d) CH2 is isolobal to Mn(CO)4
Ans. a
Sol. 
ans so on..........
Correct answer is (a)
115. MnCr2O4 is likely to have a normal spinel structure because
(a) Mn2+ will have a LFSE in the octahedral site whereas the Cr3+ will not
(b) Mn is +2 oxidation state and both the Cr and in +3 oxidation state
(c) Mn is +3 oxidation state and 1 Cr is in +2 and the other is in +3 state
(d) Cr3+ will have a LFSe in the octahedral site whereas the Mn2+ ion will not
Ans. d
Sol. 
Correct answer is (d)
116. The ground state forms of Sm3+ and Eu3+ respectively, are
(a) 7F0 and 6H5/2
(b) 6H5/2 and 7F0
(c) 2F5/2 and 5I4
(d) 7F6 and 2F7/2
Ans. b
Sol. 


Correct answer is (b)
117. The orbital interactions shown below represent

(a) CH3 – Al interactions in Al2(CH3)6
(b) B – H interactions in B2H6
(c) CH3 – Li interaction in Li4(CH3)4
(d) CH3 CH2 – Mg interactions in EtMgBr.(OEt2)2
Ans. c
Sol. 
The diagram clearly indicates the four centered – two electron interaction (4c – 2e). This takes place in  The sp3 hybrid orbital is of carbon while the three s – orbitals are of three surrounding lithium atoms.
The sp3 hybrid orbital is of carbon while the three s – orbitals are of three surrounding lithium atoms.
Correct option is (c)
118. Compounds K2Ba[Cu(NO2)6] (A) and Cs2Ba[Cu(NO2)6] (B) exhibit tetragonal elongation and tetragonal compression, respectively. The unpaired electron in A and B are found respectively, in orbitals,
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. 
Correct option is (b)
119. Reaction of Ph2PCH2CH2PPh2 with [RhC1(CO)2]2 in a 2:1 molar ratio gives a crystalline solid A. The IR spectrum of complex A shows vco at 1985 cm–1. The 31P(1H) NMR spectrum of A consists of two doublets of doublets of equal intensities (103Rh is 100% abundant and I=1/2). The structure of complex A is
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. 
IR band at 1985 cm–1 indicates terminal CO. Both P atoms are non – equivalent. Each P atom will show doublet of doublet with 
Correct option is (a)
120. The most appropriate structure for the complex  is
is
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol.  bonding in weaker than that of Pt – P bonding. When Pt – S and Pt – P bond are trans to each other then Pt – S become weaker therefore in such a situation SCN ligand tends to form bonding through M atoms of ligand as M do not form
bonding in weaker than that of Pt – P bonding. When Pt – S and Pt – P bond are trans to each other then Pt – S become weaker therefore in such a situation SCN ligand tends to form bonding through M atoms of ligand as M do not form  bonding. Hence, the most probable structure will be
bonding. Hence, the most probable structure will be

Correct option is (c)
121. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. Chemical reaction involved in the above transformation can be illustrated as
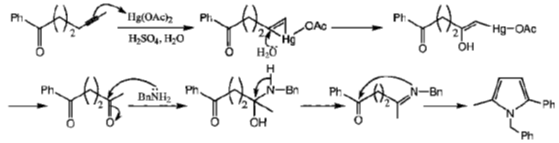
Correct option is (d)
122. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 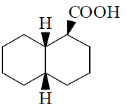
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as


since lindlar's catalyst convert
alkyne to cis alkene
Correct option is (b)
123. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. 
Correct option is (b)
124. The most suitable reagent combination of A – C, required in the following conversions are

(a) A = Li/liq. NH3; B = NaBH4, CeCL3, 7H2O; C = H2, (Ph3P)3RhCl
(b) A = Li/liq. NH3; B = NaBH4, CeCL3, 7H2O; C = H2, 10% Pd/C
(c) A = NaBH4, CeCl3, 7H2O; B = Li/liq. NH3; C = H2, (Ph3P)3RhCl
(d) A = NaBH4, CeCl3, 7H2O; B = Li/liq. NH3; C = H2, 10% Pd/C
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct option is (a)
125. The major product B formed in the following reaction sequence and overall yield of its formation are
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. 
Therefore option (b) and (d) and not possible

Since, the reaction is not happen at the stereocentre, therefore stereochemistry of the reactant will preserved in the product.
Correct option is (a)
126. An organic compound (C8H10O2), which does not change the color of ferric chloride solution, exhibited the following 1H NMR spectral data.  7.3 (1H, t, J = 8 Hz), 7.0 (1H, d, J = 8 Hz), 6.95 (1H, s), 6.9 (1H, d, J = 8 Hz) 5.3 (1H, br, s, D2O exchangeable), 4.6 (2H, s), 3.9 (3H, s). Structure of the compound is
7.3 (1H, t, J = 8 Hz), 7.0 (1H, d, J = 8 Hz), 6.95 (1H, s), 6.9 (1H, d, J = 8 Hz) 5.3 (1H, br, s, D2O exchangeable), 4.6 (2H, s), 3.9 (3H, s). Structure of the compound is
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. 
Correct option is (b)
127. Methyl 4 – oxopentanoate exhibited signals at  208, 172, 51, 37, 32 and 27 ppm in its 13C NMR spectrum. The signals due to the methoxy, Cl, C4 and C5 carbons are
208, 172, 51, 37, 32 and 27 ppm in its 13C NMR spectrum. The signals due to the methoxy, Cl, C4 and C5 carbons are
(a) OMe – 32; Cl – 208; C4 – 172; C5 – 51
(b) OMe – 51; Cl – 208; C4 – 172; C5 – 32
(c) OMe – 32; Cl – 172; C4 – 208; C5 – 51
(d) OMe – 51; Cl – 172, C4 – 208; C5 – 32
Ans. d
Sol. 
OMe  = 52 is due to attachement of carbon to O.
= 52 is due to attachement of carbon to O.
C – 5 CH3 is due to attachment of carbon to carbonyl group.
C – 4 is aliphatic carbonyl centre so above 200.
C – 1 is ester carbonyl group so below 200.
Correct answer is (d)
128. In the following reaction, the intermediate and the major product A are

(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. Chemical reaction involved in the above transformation can be illustrated as


Correct option is (d)
129. The major product formed in the sulfuric acid mediated rearrangement of the sesquiterpene saritonin A is

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as


This is a dienone – phenol type rearrangement.
Correct option is (b)
130. In the following transformation, the reagent A and the major product B, respectively, are

(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (d)
131. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as
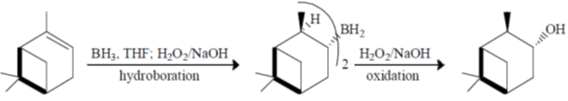

Correct option is (a)
132. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as


Correct option is (a)
133. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (b)
134. The peptide A on reaction with 1 – fluoro – 2, 4 – dinitrobenzene followed by exhaustive hydrolysis gave phenylalanine, alanine, serine and N – (2, 4 – dinitrophenyl) glycine. On the other hand, peptide A after two cycles of Edman degradation gave Phe – Ser as the product. The structure of the peptide A is
(a) Phe – Ser – Ala – Gly
(b) Phe – Ser – Gly – Ala
(c) Gly – Ala – Gly – Ala
(d) Ala – Gly – Phe – Ser
Ans. c
Sol. 2, 4 – dinotrofluorobenzene is said to be Sanger reagent and it will react with N – terminal aminoacid. The DNB – derviative of glycene indicates that, glycene is present at the N – terminal side in the peptide.
i.e.

Correct option is (c)
135. The compound (B) (labeled) is precursor for biosynthesis of the natural product A. the labeled carbons in the product A are

(a) C1, C3, C5 and Me
(b) C2, C4, C6 and Me
(c) C2, C4, C6 and COOH
(d) C1, C3, C5 and COOH
Ans. c
Sol. Correct answer is (c)
136. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. Chemical reaction involved in the above transformation can be illustrated as



Correct option is (d)
137. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Chemical reaction involved in the transformation can be illustrated as

Correct option is (a)
138. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as


Correct option is (b)
139. The conditions A-B, required for the following pericyclic reactions are
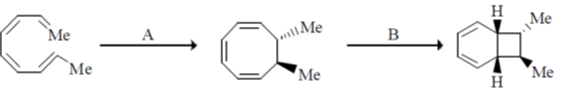
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct option is (b)
140. The number of  electrons participating and the pericyclic mode in the following reaction are
electrons participating and the pericyclic mode in the following reaction are

(a) 4 and conrotatory
(b) 4 and disrotatory
(c) 6 and conrotatory
(d) 6 and disrotatory
Ans. d
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct option is (d)
141. Stereoselective reduction of the dione A with a chiral reducing agent provides the corresponding diol B in 100% diastereoselectivity and 90% ee favoring R, R configuration. The composition of the product is

(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. % ee = 90

Hence, one diastereomer will be (90 + 5) = 95% and another will be 5%
In case of diastereomer both of stereocentre are changed.
Correct option is (d)
142. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct option is (b)
143. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as


Correct answer is (a)
144. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. 

Correct option is (a)
145. The major product formed in the following photochemical reaction is

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct option is (b)
