CSIR NET CHEMISTRY (DEC-2011)
Previous Year Question Paper with Solution.
21. Identify which of the following operators is not hermitian ?
(a) 
(b) 
(c) 
(d) x2
Ans. b
Sol.  (Hermitian) and
(Hermitian) and  (Not hermitian)
(Not hermitian)
For option (a),

It is hermitian.
For option (b),

It is Not hermitian.

Correct answer is (b)
22. The term symbol for the ground state of nitrogen atoms is
(a) 
(b) 
(c) 
(d) 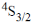
Ans. d
Sol. 
23. PA and PB denote the populations of two energy states EA and EB , and EA > EB. The correct statement when the temperature T1 > T2 is
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. Population 
Therefore at constant temperature lower the energy (E) higher will be population.

Also higher the energy value, higher will be the change in population ratio when temperature is changed from T2 to T1

24. The uncertainty in the NMR frequency of a compound in liquid state (relaxation time =1s) is 0.1 Hz. The uncertainty in the frequency (in Hz) of same compound in solid state (relaxation time = 10–4 s) is
(a) 10–4
(b) 100
(c) 1000
(d) 10–3
Ans. c
Sol. From Heisenberg uncertaintity principle,


(where  = life time or Relaxation time and
= life time or Relaxation time and  = uncertainty in frequency)
= uncertainty in frequency)

From equation (ii) and (iii)


Correct answer is (c)
25. Which one of the following conductometric titrations will show a linear increase of the conductance with volume of the titrant added up to the break point and an almost constant conductance afterwards.
(a) A strong acid with a strong base
(b) A strong acid with a weak base
(c) A weak acid with a strong base
(d) A weak acid a weak base.
Ans. d
Sol. 
When weak base is added to weak acid then salt is formed which is more dissocited w.r.t. weak acid, so conductance increases upto end point but after the end point only concentration of weak base increases which remains in almost undissociated form so does not lead to increases in conductance.
Correct answer is (d).
26. Flocculation value of K2SO4 is much less than that of KBr for Sol A. Floccultion value of CaCl2 is much less than that of NaCl for Sol B. Which of the following statements is correct?
(a) Sol A is negatively charged and Sol B is positively charged
(b) Both the sols are negatively charged.
(c) Sol A is positively charged and sol B is negatively charged
(d) Both the sols are positively charged.
Ans. c
Sol. If charge on cation is high then its flocculation value will be low for an anionic sol and if charge on anion is high then its flocculation value will be low for a cationic Sol. As K2SO4has low flocculation value than KBr (due to SO42– has higher are charge than Br–) ; so sol. A is cationic in nature.
On sol B, CaCl2 has low flocculation value than NaCl due to higher the change on calcium ion than sodium ion. So sol B is negatively charged.
Correct answer is (c).
27. For a system of constant composition, the pressure (P) is given by.
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. 
Correct answer is (b)
28. The value of d111 in a cubic crystal is 325.6 pm. The value of d333 is
(a) 325.6 pm
(b) 976.8 pm
(c) 108.5 pm
(d) 625.6 pm
Ans. c
Sol. 
Correct answer is (c)
29. The symmetry point group of ethane in its staggered conformation is
(a) C3v
(b) D3d
(c) D3h
(d) S6
Ans. b
Sol. Correct answer is (b)
30. TFor the reaction C2H4 (g) + 3O2 (g)  2CO2(g) + 2H2O (l), The value of
2CO2(g) + 2H2O (l), The value of  (in kJ) at 300 K and 1 bar is
(in kJ) at 300 K and 1 bar is
(a) –5.0
(b) 0.0
(c) 2.5
(d) 5.0
Ans. a
Sol. 
Correct answer is (a)
31. The sodium D lines are due to  transitions. The splitting due to spin-orbit coupling in 2P state of the sodium atom is
transitions. The splitting due to spin-orbit coupling in 2P state of the sodium atom is
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. 

n = splitting due to spin orbital coupling = 
Correct answer is (b)
32. The rate constant of a unimolecular reaction was 2.66 × 10–3 s–1 and 2.2 × 10–1 s–1 at T = 120 K and 360 K respectively. The rate constant (in s–1 units) at 240 K would be
(a) 2.4 × 10–2
(b) 2.4 × 10–1
(c) 4.8 × 10–2
(d) 1.8 × 10–3
Ans. a
Sol. If k1 and k2 are rate constant at temperature T1 and T2 respectively and  is temperature coefficient, then these are related by the expression
is temperature coefficient, then these are related by the expression

Now at temperature 240 K, rate constant (k3)

Correct answer is (a)
33. For a potentiometric titration, in the curve of emf (E) vs volume (V) of the titrant added, the equivalence point is indicated by
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. In potentiometric titration the rate of change in slope of E vs V graph has maximum at the equivalence point

And as  can be positive or negative. But
can be positive or negative. But  will always be greater than zero.
will always be greater than zero.
Correct answer is (c)
34. The osmotic pressure  of a polymer sample at different concentrations (c) was measured at T(K). A plot of
of a polymer sample at different concentrations (c) was measured at T(K). A plot of  versus c gave a straight line with slope (m) and intercept (c´). The number average molecular weight of the polymer is (R = gas constant).
versus c gave a straight line with slope (m) and intercept (c´). The number average molecular weight of the polymer is (R = gas constant).
(a) 
(b) 
(c) RT
(d) mRT
Ans. b
Sol. Osmotic pressure, 
where C1 = concentration of polymer in mol L–1 unit and B = constant.
When concentration is expressed in terms of 'c' (gL–1) then, C1 = Mc

Correct answer is (b)
35. The concentration of a reactant undergoing decomposition was 0.1, 0.08 and 0.067 mol L–1 after 1.0, 2.0 and 3.0 hr respectively. The order of the reaction is
(a) 0
(b) 1
(c) 2
(d) 3
Ans. c
Sol. 
Above data first with second order rate law expression for the reaction

From data (i) and (ii)

From data (i) and (iii)

As the value of k is constant so it is second order reaction
Correct answer is (c)
36. A particle is constrained in one dimensional box of length 2a with potential  x < –a, x > a and V(x) = 0; –a < x < a. Energy difference between levels n = 3 and n = 2 is
x < –a, x > a and V(x) = 0; –a < x < a. Energy difference between levels n = 3 and n = 2 is
(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. For 1-D box, the energy is



Correct answer is (d)
37. In the 19F NMR spectrum of PF5, the number of signals and multiplicity, at room temperature are
(a) one, singlet
(b) one, doublet
(c) two, doublet
(d) two, singlet
Ans. b
Sol. At room temperature due to rapid interconversion of axial and equatorial F atoms, all the fluorine will be equivalent. Hence 19F NMR of PF5 at room temperature will appear as doublet of 5F.
Correct answer is (b).
38. The correct statement regarding closo-{BnHn} species is
(a) it always has –2 charge
(b) it always has +2 charge
(c) it is a neutral species
(d) It is more reactive than nido arachno-, and hypo-boranes
Ans. a
Sol. Closo BnHn species has always –2 charge.
Correct answer is (a)
39. Lewis acidity of BCl3, BPh3 and BMe3 with respect to pyridine follows the order
(a) BCl3 > BPh3 > BMe3
(b) BMe3 > BPh3 > BCl3
(c) BPh3 > BMe3 > BCl3
(d) BCl3 > BMe3 > BPh3
Ans. c
Sol. 
 B-attracts B-CH3 electron (Hyperconjugation)
B-attracts B-CH3 electron (Hyperconjugation)
 B and C both are sp2-hybridised. No shifting of electrons (Resonance).
B and C both are sp2-hybridised. No shifting of electrons (Resonance).
BPh3 > BMe3 > BCl3
Correct answer is (c)
40. Superoxide dismutase contains the metal ions
(a) Zn(II) and Ni(II)
(b) Cu(II) and Zn (II)
(c) Ni(II) and Co(III)
(d) Cu(II) and Fe(III)
Ans. b
Sol. Correct answer is (b)
41. The number of antibonding electrons in NO and CO according to MO theory are respectively.
(a) 1, 0
(b) 2, 2
(c) 3, 2
(d) 2, 3
Ans. a
Sol. Energy level for NO, 
Number of electrons in antibonding MO = 1
Energy level for CO, 
No electron is present in antibonding MO.
So, number of antibonding electron in CO is zero
Correct answer is (a)
42. The correct combination of metal, number of carbonyl ligands and the charge for a metal carbonyl complex [M(CO)x]z– that satisfied the 18 electron rule is
(a) M = Ti, x = 6, z = 1
(b) M = V, x = 6, z = 1
(c) M = Co, x = 4, z = 2
(d) M = Mo, x = 5, z = 1
Ans. b
Sol. M = V, x = 6, z = 1
i.e. 
Correct answer is (b).
43. Among the following pairs
Those in which the first ionization energies differ by more than 300 kJ mole–1 are:
(a) (1) and (3) only
(b) (1) and (2) only
(c) (2) and (3) only
(d) (3) and (4) only
Ans. b
Sol. (A) IE1 for O and S are 1314 and 1000 kJ mol–1.
difference = 1314 – 1000 = 314
(B) For N and P IE1 are 1402 and 1012.
difference = 1402 – 1012 = 390
(C) For P and As IE1 are 1012 and 947
difference = 1012 – 947 = 165 (less 300 kJ mol–1).
(D) Also for IE1 difference is less than 300 kJ mol–1.
Correct answer is (b)
44. The stable cyclopentadienyl complex of beryllium is
(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. Because main group element follows octet rule.
2 + 5 + 1 = 8
Correct answer is (d)
45. The reaction between NH4Br and Na metal in liquid ammonia (solvent) results in the products
(a) NaBr, HBr
(b) NaBr, H2
(c) H2, HBr
(d) HBr, H2
Ans. b
Sol. 


Correct answer is (b)
46. The material that exhibits the highest electrical conductivity among the following sulfur-nitrogen compounds is
(a) S4N4
(b) S7NH
(c) S2N2
(d) (SN)x
Ans. d
Sol. (SN)x  because it is a giant cross linked molecule and have mobile electrons.
because it is a giant cross linked molecule and have mobile electrons.
Correct answer is (d)
47. Uranium fluorides co-precipitate with
(a) CaF2
(b) AgF
(c) LiF
(d) MgF2
Ans. a
Sol. Correct answer is (a)
48. The acid-base indicator (HIn) shows a colour change at pH 6.40 when 20% of it is ionized. The dissociation constant of the indicator is
(a) 9.95 × 10–8
(b) 3.95 × 10–6
(c) 4.5 × 10–8
(d) 6.0 × 10–8
Ans. a
Sol. 
Correct answer (a)
49. The actual magnetic moment shows a large deviation from the spin-only formula in the case of
(a) Ti3+
(b) V3+
(c) Gd3+
(d) Sm3+
Ans. d
Sol. Sm3+  due to spin-orbital coupling
due to spin-orbital coupling
Correct answer is (d)
50. The complex that absorbs light of shortest wavelength is
(a) [CoF6]3–
(b) [Co(H2O)6]3+
(c) [Co(NH3)6]3+
(d) [Co(OX)3]3– (OX = C2O42–)
Ans. c
Sol. 
 or lowest value of wavelength is absorbed
or lowest value of wavelength is absorbed
Correct answer is (c).
51. Two  particles having speeds S1 and S2 have kinetic energies 1 and 2 MeV respectively; the relationship between S1 and S2 is
particles having speeds S1 and S2 have kinetic energies 1 and 2 MeV respectively; the relationship between S1 and S2 is
(a) S1 = 2S2
(b) S2 = 2S1
(c) 
(d) 
Ans. c
Sol. 

Correct answer is (c)
52. Green coloured Ni(PPh2Et)2Br2, has a magnetic moment of 3.20 B.M. The geometry and the number of isomers possible for the complex respectively are
(a) Square planar and one
(b) tetrahedral and one
(c) Square planer and two
(d) tetrahedral and two
Ans. b
Sol. Ni(PPh2Et)2Br2; 
Number of unpaired electrons = 2.
Thus this complex is tetrahedral. Tetrahedral complexes show only one isomer.
Magnetic moment corresponding to two unpaired electrons = 2.9 B.M. The hesh values 3.20 BM is due to spin-orbit coupling.
Correct answer is (b)
53. The chemiluminescence method for determining NO in environmental sample is based on formation of NO2* (excited) which is generally generated by reacting NO with
(a) O2
(b) 
(c) O3
(d) 
Ans. a
Sol. 
54. In the IR spectrum, carbonyl absorption band for the following compound appears at

(a) 1810 cm–1
(b) 1770 cm–1
(c) 1730 cm–1
(d) 1690 cm–1
Ans. b
Sol. C = O stretching frequency for simple ester 1750 – 1725 cm–1.
For six membered lactone = 1750 – 1735 cm–1
For five membered lactone = 1795 – 1760 cm–1
Correct answer is (b)
55. Among the following compounds, the formyl anion equivalent is
(a) acetylene
(b) nitromethane
(c) ethyl chloroformate
(d) 1, 4-dithiane
Ans. b
Sol. Nitromethane is used as a synthetic equivalent for formyl anion.
Correct answer is (b)
56. In the following concerted reaction, the product is formed by a

(a)  disrotatory electrocyclization
disrotatory electrocyclization
(b)  disrotatory electrocyclization
disrotatory electrocyclization
(c)  conrotatory electrocyclization
conrotatory electrocyclization
(d)  conrotatory electrocyclization
conrotatory electrocyclization
Ans. a
Sol. According to the given product stereochemistry, the product is formed by  disrotatory electrocyclization
disrotatory electrocyclization

Correct answer is (a)
57. A suitable reagent combination for carrying out the following conversion is

(a) trimethyl orthoacetate and p-toluenesulfonic acid
(b) trimethyl ortho acetate and sodium hydroxide
(c) 2-methoxypropene and p-toluenesulfonic acid
(d) 2-methoxypropene and sodium hydroxide
Ans. c
Sol. Chemical reaction involved in the above transformation can be illustrated as


Correct answer is (c)
58. The IUPAC name of the following compound is

(a) (R)-3-(prop-2-enyl) hex-5-ynoic acid
(b) (S)-3-(prop-2-enyl) hex-5-ynoic acid
(c) (R)-3-(prop-2-enyl) hex-5-enoic acid
(d) (S)-3-(prop-2-ynyl) hex-5-enoic acid
Ans. b
Sol. IUPAC name of above compound can be written using following keynotes
(1) carboxylic group has to be given highest priority
(2) keeping the chain tripple bond is parent chain.

(S)-3-(prop-2 enyl) hex-5-ynoic acid
Correct answer is (b).
59. In the mass spectrum of dodecahedrane (C20H20), approximate ratio of the peaks at m/z 260 and 261 is
(a) 1 : 1
(b) 5 : 1
(c) 10 : 1
(d) 20 : 1
Ans. b
Sol. In C20H20 due to 20 carbons, there will be 20 × 1.1% probability of one 13C in the molecule, where 1.1% is natural abundance of 13C with molecular ion, there will be 22% of M + 1 signal. Hence if 260 is 100% and 261 will be 22%.

60. The reaction given below proceeds through

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. 
Correct answer is (a)
61. Among the following drugs, the anticancer agents is
(a) Captopril
(b) chloroquine
(c) camptothecin
(d) ranitidine
Ans. c
Sol. Correct answer is (c)
62. The reaction that involves the formation of both C-C and C-O bonds is
(a) Diels-Alder reaction
(b) Darzen's glycidic ester condensation
(c) Aldol reaction
(d) Beckmann rearrangement
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (b)
63. Among A-C, the aromatic compound are

(a) A, B and C
(b) A and B only
(c) B and C only
(d) A and C only
Ans. c
Sol. Since in case of A the hydrogens present at 1st and 6th position repel each others. Hence planarity is distorts. While in case of B and C this type of planarity is maintained

Correct answer is (c)
64. In the following Markownikov addition reaction, the products A and B are

(a) homomers
(b) enantiomers
(c) diastereomers
(d) regioisomers
Ans. c
Sol. Chemical reaction involved in the above transformation can be illustrated as

Compound A and B are diastereomers due to difference in absolute configuration at carbon 2 and 3.
Correct answer is (c)
65. The major product formed in the following reaction is

(a) 
(b) 
(c) 
(d) 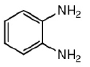
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (a)
66. Among A-C, the compounds which can exhibit optical activity are

(a) A, B and C
(b) A and B only
(c) A and C only
(d) B and C only
Ans. c
Sol. Compound B can be represented in fisher projection as shown below

Here in fisher projection it contain plane of symmetry passes through mid of C2-C3. Hence it is optically inactive.
Correct answer is (c)
67. The major product formed in the following reaction is

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (a)
68. An organic compound (MF : C8H10O) exhibited the following 1H NMR spectral data :  2.5 (3 H,s), 3.8 (3H, s), 6.8 (2 H, d, J 8 = Hz), 7.2 (2H, d, J = 8 Hz) ppm. The compound among the choices, is
2.5 (3 H,s), 3.8 (3H, s), 6.8 (2 H, d, J 8 = Hz), 7.2 (2H, d, J = 8 Hz) ppm. The compound among the choices, is
(a) 4-ethylphenol
(b) 2-ethylphenol
(c) 4-methylanisole
(d) 4-methylbenzyl alcohol
Ans. c
Sol. Characterization of organic compound can be done as

 indicates para hetro disubstituted benzene ring.
indicates para hetro disubstituted benzene ring.

Correct answer is (c)
69. With respect to electrophilic aromatic substitution, reactivity order of pyrrole, pyridine and indole is
(a) indole > pyrrole > pyridine
(b) pyrrole > pyridine > indole
(c) pyrrole > indole > pyridine
(d) indole > pyridine > pyrrole
Ans. c
Sol. pyrrole > Indole > Pyridine
Pyrrole is considered to be  centre where in HOMO is at higher energy level where as Pyridine is
centre where in HOMO is at higher energy level where as Pyridine is  - sin k centre whose HOMO is at low energy level, and pyridine gives nucleophilic substitution reaction rather then electrophilic substitution reaction.
- sin k centre whose HOMO is at low energy level, and pyridine gives nucleophilic substitution reaction rather then electrophilic substitution reaction.

Correct answer is (c)
70. The most appropriate reagent suitable for the conversion of 2-octyne into trans-2-octene is
(a) zinc and acetic acid
(b) 10% Pd/C
(c) lithium in liquid ammonia
(d) hydrazine hydrate
Ans. c
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (c)
71. Consider a n-type semiconductor whose Ev = 0, Ec = 2.0 eV and Ed = 1.98 eV. The correct statement among the following is
(a) Ef = 1 eV and is independent of T
(b) Ef = 1.99 eV and remains independent of T
(c) Ef = 1.99 eV and increases towards 2.0 eV with increase of T
(d) Ef = 1.99 eV and decreases with increase of T.
Ans. d
Sol. 
For fermi level lies exactly half any between the bottom or conduction band and the donor level. But as temperature is increased fermi level drops (i.e. Ef decreases).
Correct answer is (d)
72. Reaction of Fe(CO)5 with OH– leads to complex A which on oxidation with MnO2 gives B. Compounds A and B respectively are
(a) [HFe(CO)4]– and Fe3(CO)12
(b) [Fe(CO)5(OH)]– and Fe2(CO)9
(c) [Fe(CO)4]2– and Mn2(CO)10
(d) [HFe(CO)4]– and Fe2O3
Ans. a
Sol. Correct answer is (a)
73. For the reaction H2O(g) + C(graphite)  CO(g) + H2O(g), the variation of energy parameter
CO(g) + H2O(g), the variation of energy parameter  of the reaction over a large temperature range is shown below. The correct identification of the curves is given by
of the reaction over a large temperature range is shown below. The correct identification of the curves is given by

(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. 
As the number of mole of gaseous product is more than reactant 
So there is overall increase in entropy

 will increase with increase in temperature
will increase with increase in temperature 
Enthalpy of reaction  is independent of temperature
is independent of temperature

So with increases of temperature  decreases because
decreases because 
Correct answer is (d)
74. A Sodalite cage in zeolites is
(a) a truncated tetrahedron
(b) an icosahedron
(c) a truncated octahedron
(d) a dodecahedron
Ans. c
Sol. Correct answer is (c)
75. Two moles of a nonvolatile solute is dissolved in 48 mol of water and the resultant solution has a vapour pressure of 0.0392 bar at 300 K. If the vapour pressure of pure water at 300 K is 0.0400 bar, the activity coefficient of water in the solution is
(a) 0.96
(b) 0.98
(c) 1.00
(d) 1.02
Ans. d
Sol. From Roult's law, Relative lowering of vapour pressure = activity of solute


Correct answer (d)
76. The final product (s) of the reaction P(OR)3 + R´X is/are
(a) R´PO(OR)2 and RX
(b) [R´PO(OR)2]X
(c) [R´RPO2(OR)]X
(d) ROR´ and P(OR)2 X
Ans. a
Sol. 
Because P–O bond very strong affinity very high not easily break. So, R–O bond break.
Correct answer is (a)
77. 1 mol of CO2, 1 mol of N2 and 2 mol of O2 were mixed at 300 K. The entropy of mixing is
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Since, we know that for the entropy of mixing is


Correct answer is (a)
78. For the eigenstates of the hydrogen atom, which of the following relations between the expectation value of kinetic energy (T) and potential (V) holds true?
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. For H-atom, virial theorem
(i) V = 2E (ii) V = –2T (iii) T = –E
Where, T = total energy
T = kinetic energy
V = potential energy
 <V> = –2 <T>
<V> = –2 <T>
Correct answer is (b).
79. For the liquid  vapour equilibrium of a substance
vapour equilibrium of a substance  at 1 bar and 400 is 8 × 10–3 bar K–1. If the molar volume in the vapour form is 200 L mol–1 and the molar volume in the liquid form is negligible, the molar enthalpy of vapourisation is (1.0 bar L = 100 J)
at 1 bar and 400 is 8 × 10–3 bar K–1. If the molar volume in the vapour form is 200 L mol–1 and the molar volume in the liquid form is negligible, the molar enthalpy of vapourisation is (1.0 bar L = 100 J)
(a) 640 kJ mol–1
(b) 100 kJ mol–1
(c) 80 kJ mol–1
(d) 64 kJ mol–1
Ans. d
Sol. 
Or, 
= 640 × 100 J mol–1 
= 64 kJ mol–1
Correct answer is (d)
80. The correct order of acidity among the following species is
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Higher the charge and smaller size of the central metal cation increases the acidic strength of hydrated complex because it polarizes O–H band of H2O easily.
In [Sc(H2O)6]3+, Sc is in +3 oxidation state and has smallest size.
Therefore, in size of depositive ions increase in the order Ni2+ < Mn2+. Na+ has lowest oxidation state and largest size.
Correct answer is (b)
81. The Langmuir adsorption isotherm is given by  where P is the pressure of the adsorabate gas. The Langmuir adsorption isotherm for a diatomic gas A2 undergoing dissociative adsorption is
where P is the pressure of the adsorabate gas. The Langmuir adsorption isotherm for a diatomic gas A2 undergoing dissociative adsorption is
(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. For non-dissociative Langmuir adsorption,

Correct answer is (d)
82. The standard electrode potentials (Eº) of Fe3+ / Fe2+ and Fe2+ / Fe electrodes are +0.77 V and –0.44 V respectively at 300 K. The Eº of Fe3+ / Fe electrode at the same temperature is
(a) 1.21 V
(b) 0.33 V
(c) –0.11 V
(d) –0.04 V
Ans. d
Sol. 
Adding above half cell reactions

Correct answer is (d)
83. Which of the following is true for the radial part of the hydrogen atom wavefunctions  (n principal quantum number) and the nodes associated with them?
(n principal quantum number) and the nodes associated with them?
(a) The radial part of only s function is non-zero at the origin and has (n – 1) nodes.
(b) The radial part of s function is zero at the origin and has n number of nodes.
(c) All radial function have values of zero at the origin and have (n – 1) nodes.
(d) The radial parts of all s functions are zero at the origin and have no nodes.
Ans. a
Sol. Number of radial nodes = n – l – 1
For s-function l = 0
Therefore, number of nodes = n – 0 – 1 = n – 1
Every function either s, p, d or f have non zero at the origin.
Correct answer is (a)
84. For non-degenerate perturbation theory for ground state, with  as zeroth order energy,
as zeroth order energy,  as the first order perturbation correction and E0 as the exact energy, which of the following is true?
as the first order perturbation correction and E0 as the exact energy, which of the following is true?
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. According to "The variation principle" If any orbitary wavefunction is used to calculate the energy, the value calculated is never less the true energy.
 Energy calculated by using an arbitary wavefunction and applying perturbation will always be greater than or equal to true energy.
Energy calculated by using an arbitary wavefunction and applying perturbation will always be greater than or equal to true energy.

Correct answer is (c)
85. Observe the following electronic transition of a diatomic molecule.


The allowed transitions are
(a) A and C only
(b) B and D only
(c) A, B and C only
(d) A, C and D only
Ans. c
Sol. For allowed transitions are 
Correct answer is (b)
86. An excited triplet state wave function of hydrogen molecule with the electronic configuration  has the following space part
has the following space part
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. According to the pauli exclusion principle, the wavefunction of fermions (here electrons) must be antisymmetric 
For a triples state, spin wavefunctions can be  which are symmetric in nature. As
which are symmetric in nature. As  So, for
So, for  to be antisymmetrical w.r.t. electron exchange,
to be antisymmetrical w.r.t. electron exchange,  must be antisymmetric because for triplet state
must be antisymmetric because for triplet state  is symmetric so,
is symmetric so, 
Correct answer is (c).
87. The NMR spectrum of AX3 exhibits lines at  = 2.1 and 2.3 ppm (for X type protons) and
= 2.1 and 2.3 ppm (for X type protons) and  = 4.1, 4.3, 4.5 and 4.7 ppm (for A type protons), measured from TMS with an instrument operating at 100 MHz. The chemical shift (in ppm) of A and X protons and coupling constant (in Hz) are respectively.
= 4.1, 4.3, 4.5 and 4.7 ppm (for A type protons), measured from TMS with an instrument operating at 100 MHz. The chemical shift (in ppm) of A and X protons and coupling constant (in Hz) are respectively.
(a) 4.4, 2.2 and 20
(b) 2.2, 4.4 and 10
(c) 2.2, 4.4 and 5
(d) 4.3, 2.1 and 20
Ans. a
Sol. In AX3 A proton spectra will be a quartet (Intensity ratio 1 : 3 : 3 : 1) at  = 4.1, 4.3, 4.5 and 4.7 ppm and X proton spectra will be a doublet (Intensity ratio 1 : 1)
= 4.1, 4.3, 4.5 and 4.7 ppm and X proton spectra will be a doublet (Intensity ratio 1 : 1)

88. The character table of the C2v point group is given below:

The two function  and
and  (where pk is the p-orbital on the kth atom of cis-butadiene and
(where pk is the p-orbital on the kth atom of cis-butadiene and  is the molecular plane) belong to
is the molecular plane) belong to
(a) A1 and A2 respectively
(b) Both A2
(c) Both B2
(d) B1 and B2 respectively
Ans. c
Sol. 


Both the molecular orbitals belongs to B2.
Correct answer is (c).
89. If  denotes the characteristic temperature of rotation then the magnitude of
denotes the characteristic temperature of rotation then the magnitude of  (assume the bond lengths to be the same for all the molecules) is
(assume the bond lengths to be the same for all the molecules) is
(a) 2/3
(b) 3/2
(c) 8/9
(d) 9/8
Ans. c
Sol. 

Correct answer is (c)
90. The overall reaction for the passage of 1.0 faraday of charge in the following cell

is given by (t denotes the transport numbers)
(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Let one faraday of electricity is withdrawn from the cell

The net effect is obtained by adding the above four changes, which gives

Correct answer is (a)
91. A system consisting of 4 identical and distinguishable particle, each possessing three available states of 1, 2 and 3 units, has 10 units of energy. The number of ways, W, in which these conditions are satisfied is
(a) 2
(b) 4
(c) 6
(d) 10
Ans. d
Sol. In order to have 10 unit total energy only 2 combinations of selecting particles are possible
(i) One particle with 1 unit energy and 3 particles with 3 unit energy each
Therefore, number of ways possible = 4C1 × 3C3 = 4 × 1 = 4 ways
(Because first particle can be selected in 4 ways next 3 particles from remaining 3-particles can be selected in only 1 way)
(ii) 2 particles with 2 unit energy each and 2 particles with 3 unit energy each
Therefore, number of ways possible = (selection of 2 particles out of 4-particles) × (selecting of 2 particles out of remaining 2-particles) = 4C2 × 2C2 = 6 × 1 = 6 ways
Therefore total number of ways = 4 + 6 = 10 ways.
Correct answer is (d)
92. The molar conductivities at infinite dilution  for Na2SO4, K2SO4, KCl, HCl and HCOONa at 300 K are 260, 308, 150, 426 and 105 S cm–1 mol–2 respectively. Hence
for Na2SO4, K2SO4, KCl, HCl and HCOONa at 300 K are 260, 308, 150, 426 and 105 S cm–1 mol–2 respectively. Hence  or formic acid in the same unit and at the same temperature is
or formic acid in the same unit and at the same temperature is
(a) 381
(b) 405
(c) 429
(d) 531
Ans. b
Sol. 
Correct answer is (b).
93. If the displacement vectors of all atoms in cis-butadiene are taken as the basis vectors the characters of the reducible representation of  (molecular plane) and
(molecular plane) and 
(a) 30, 10, 30, 0
(b) 30, 0, 10, 0
(c) 30, 20, 0, 0
(d) 30, 0, 20, 0
Ans. b
Sol. Basis set is displacement of vectors of all atoms = 3 N coordinates.

Correct answer is (b)
94. In least square fitting of a data set {XiVi} to the equation Y = A.X, the regression coefficient (A) is estimated by
(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. For data set {Xi Yi}. When equation is Y = AX + B
Its deviatinos are token from the middle period so that  the value of A and B can be directly written as
the value of A and B can be directly written as 
Correct answer is (b).
95. At any temperature for the following reaction (D and T are deuterium and tritium respectively) correct statement is


(a) A is fastest
(b) B is fastest
(c) C is fastest
(d) All the above reactions have the same rate constant.
Ans. a
Sol. Heavier isotope contains stronger bonding. Stronger the bonding reaction rate is slow. But in lighter isotope bonding is weak, so reaction rate is faster.
Correct answer is (a)
96. An example of a relaxation method of measuring rates is:
(a) Spectroscopic monitoring of product concentration.
(b) Stopped flow technique
(c) Temperature jump experiments
(d) Measurements of spectral line widths
Ans. c
Sol. When equilibrium reactions are disturbed from their equilibrium condition via either T-Jump or pH-Jump then measurement of relaxation time leads to the study of reaction rate.
Correct answer is (c).
97. The overall rate of the following complex reaction,

by steady state approximation would be
(a) K1K2k3[A]3[B]
(b) K2K1k3[A][B]3
(c) K1K2k3[A][B]2
(d) K1K2k3[A][B]
Ans. a
Sol. Overall reaction can be obtained by simple addition of mechanism steps,
So, 

As A2 and C are not appearing in the overall reaction so they are intermediates. As equilibrium is fast so concentration of [A2] and [C] can be determined using equilibrium conditions.


From reaction (1), (2) and (3), we get 
Correct answer is (a).
98. The vibrational energy levels,  and
and  of a diatomic molecule are separated by 2143 cm–1. Its anharmonicity
of a diatomic molecule are separated by 2143 cm–1. Its anharmonicity  is 14 cm–1. The values of
is 14 cm–1. The values of  (in cm–1) and first overtone (cm–1) of this molecule are respectively.
(in cm–1) and first overtone (cm–1) of this molecule are respectively.
(a) 2143 and 4286
(b) 2157 and 4286
(c) 2157 and 4314
(d) 2171 and 4258
Ans. a
Sol. 
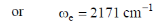
First overtone more number is given by,

Correct answer is (a).
99. The addition polymerization of M (monomer) involves the following stages:
(I = initiator, R = free radical)

The rate constant for free radical formation is 2 × 10–3 s–1. The initial concentration of initiator is 10–3 mol dm–3. The overall rate of the reaction is 4 × 10–3 mol dm–3 s–1. Assuming steady state approximation for free radical, the kinetic chain length is:
(a) 2000
(b) 8 × 109
(c) 20
(d) 200
Ans. a
Sol. Kinetic chain length = 

Correct answer is (a)
100. The electronic spectrum of [CrF6]3– shows three bands at 14,900 cm–1, 22400 cm–1 and 34,800 cm–1. The value of  in this case is
in this case is
(a) 5,500 cm–1
(b) 14,900 cm–1
(c) 22,400 cm–1
(d) 34,800 cm–1
Ans. b
Sol. For d3 and d8, the lowest transition gives the value of 


101. Among the following pairs, those in which both species have similar structures are:


(a) A and B only
(b) A and C only
(c) A, B and C only
(d) B, C and D only
Ans. b
Sol. 
Correct answer is (b)
102. The number of metal-metal bonds in the dimers, [CpFe(CO)(NO)]2 and [CpMo(CO)3]2 respectively, are
(a) two and two
(b) two and three
(c) one and two
(d) zero and one
Ans. d
Sol. [CpFe(CO)(NO)]2
Step-1: Determine the total valence electron (TVE) = A
A = [5 + 8 + 2 + 3] × 2 = [18] × 2 = 36
Step-2: B = (n × 18) – A, where n = number of metal.
B = (2 × 18) – 36 = 36 – 36 = 0
No metal-metal bond found.
[CpMo(CO)3]2
Step-1: A = [5 + 6 + 2 × 3] × 2 = [17] × 2 = 34
Step-2: B = (n × 18) – A = 2 × 18 – 34 = 36 – 34 = 2
Step-3: B/2 = gives the total number of M-M bonds in the complex
B = 2 and B/2 = 2/2 = 1
In this complex, the total number of M-M bonds is one.
Correct answer is (d).
103. The reduction of nitrogen to ammonia, carried out by the enzyme nitrogenase, needs,
(a) 2 electrons
(b) 4 electrons
(c) 6 electrons
(d) 8 electrons
Ans. d
Sol. 
Correct answer is (d)
104. In the titration of 50 mL of 0.1 M HCl with 0.1 M NaOH using methyl orange as an indicator, the end point (color change) occurs as pH reaches 4.0. The titration error is:
(a) –0.2%
(b) –84.7%1
(c) +0.2%
(d) +84.2%1
Ans. a
Sol. Correct answer is (a)
105. The styx code of B4H10 is
(a) 4120
(b) 4220
(c) 4012
(d) 3203
Ans. c
Sol. 

Correct answer is (c)
106. Match list 1 (compounds) with list II (structures), and select the correct answer using the codes using below

(a) A-ii, B-iii, C-i
(b) A-iii, B-i, C-ii
(c) A-ii, B-i, C-iii
(d) A-i, B-ii, C-iii
Ans. c
Sol. XeO4 = tetrahedral, BrF4– = Square Planar, SeCl4 = distorted Tetrahedral
Correct answer is (c)
107. In the trans-PtCl2L(CO) complex, the CO stretching frequency for L = NH3, pyridine, NMe3 decreases in the order.
(a) pyridine > NH3 > NMe3
(b) NH3 > pyridine > NMe3
(c) NMe3 > NH2 > pyridine
(d) pyridine > NMe3 > NH3
Ans. a
Sol. Order of  donor ability NMe3 > NH3 > Py
donor ability NMe3 > NH3 > Py
Order of  Py > NH3 > NMe3
Py > NH3 > NMe3
Correct answer is (a)
108. For the nuclear reactions.

(Given masses: 8Be = 8.005300, 4He = 4.002603 and  The correct statement is
The correct statement is
(a) (A) and (B) are both spontaneous fission processes.
(b) (A) is spontaneous fission but (B) is not
(c) (B) is spontaneous fission but (A) is not.
(d) Both (A) and (B) are not spontaneous fission processes.
Ans. b
Sol. 
There is mass loss. Thus, energy evolve.
Therefore, exothermic

No mass loss, no energy released
Therefore, endothermic
Correct answer is (b)
109. A metal ion that replace manganese (II) ion in mangano-proteins without changing its function, is
(a) Fe(II)
(b) Zn(II)
(c) Mg(II)
(d) Cu(II)
Ans. c
Sol. Correct answer is (c)
110. In 57Fe* Mossbauer experiment, source of 14.4 keV (equivalent to 3.48 × 1012 MHz) is moved towards absorber at a velocity of 2.2 mm s–1. The shift in frequency of the source for this sample is:
(a) 35.5 MHz
(b) 25.5 MHz
(c) 20.2 MHz
(d) 15.5 MHz
Ans. b
Sol. Frequency shift  where V = relatime velocity of source and observer
where V = relatime velocity of source and observer
 frequency of emitted radiation
frequency of emitted radiation

Correct answer is (b)
111. Bayer's process involves
(a) Synthesis of B2H6 from NaBH4
(b) Synthesis of NaBH4 from borax
(c) Synthesis of NaBH4 from B2H6
(d) Synthesis of B3N3H6 from B2H6
Ans. b
Sol. 
Correct answer is (b)
112. A true statement about base hydrolysis of [Co(NH3)5Cl]2+ is
(a) It is a first order reaction
(b) The rate determining step involves the dissociation of chloride in [Co(NH3)4(NH2)Cl]+
(c) The rate is independent of the concentration of the base
(d) The rate determining step involves the abstraction of a proton from [Co(NH3)5Cl]2+
Ans. b
Sol. Substitution nucleophilic unimolecular conjugate base mechanism.

Correct answer is (b)
113. The catalyst involved in carrying out the metathesis of 1-butene to give ethylene and 3-hexene is
(a) 
(b) Na2PdCl4
(c) Co2(CO)8, H2
(d) RhCl(PPh3)3
Ans. a
Sol. Correct answer is (a).
114. The correct order of d-orbital splitting in a trigonal bipyramidal geometry is
(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. 
Correct answer is (d)
115. For the following outer sphere electron transfer reactions.

the rate constants are 10–6 M–1 s–1 and 8.2 × 102 M–1s–1 respectively. This difference in the rate constants is due to
(a) A change from high spin to low spin in Co* and high spin to low spin in Ru
(b) A change from high spin to low spin in Co* and low spin to high spin Ru*
(c) A change from low spin to high spin in Co* and the low spin state remains unchanged in Ru
(d) A change from low spin to high spin in Co* and high spin to low spin in Ru*
Ans. c
Sol. 
Correct answer is (c)
116. The greater stability of  compared to that of ((CH3)2CH – CH2 – )4Ti(B) is due to
compared to that of ((CH3)2CH – CH2 – )4Ti(B) is due to
(a) Hyperconjugation present in complex (A)
(b)  -hydride elimination is not possible in complex (A)
-hydride elimination is not possible in complex (A)
(c) Steric protection of titanium from reactive species in complex (A)
(d) The stronger nature of Ti-C bond in complex (A)
Ans. b
Sol. 
Correct answer is (b)
117. The coordination number and geometry of cerium in [Ce(NO3)6]2– are respectively,
(a) 6 and octahedron
(b) 6 and trigonal prism
(c) 8 and cubic
(d) 12 and icosahedron
Ans. d
Sol. For Ce4+ ions NO3– behaves as bidentate ligand. Thus C.N. for [Ce(NO3)6]2– is 12. and shape corresponding to C.N. 12 is icosahedron.
Correct answer is (d)
118. A compound A having the composition FeC9H8O3 shows one signal at 2.5 ppm and another one around 5.0 ppm in its 1H NMR spectrum. The IR spectrum of this compound shows two bands around and 1680 cm–1. The compound follows the 18 electron rule of the following statements for A, the correct one is/are
(A) It has  group.
group.
(B) It has a terminal CO ligand
(C) it has a CH3 ligand
(D) It has Fe-H bond
(a) A and B only
(b) C only
(c) A and C only
(d) B and D only
Ans. a
Sol. 
Correct answer is (a)
119. In bacterial rubredoxin, the number of iron atoms, sulfur bridges and cysteine ligands are
Fe atom Sulfer bridge Cysteine
(a) 4 4 4
(b) 2 2 4
(c) 2 2 2
(d) 1 0 4
Ans. d
Sol. 
Correct answer is (d)
120. In the following reaction, the product formed and the mechanism involved are

(a) A is  and is formed by addition-elimination mechanism
and is formed by addition-elimination mechanism
(b) A is  and is formed by benzyne mechanism
and is formed by benzyne mechanism
(c) A is  and is formed by benzyne mechanism
and is formed by benzyne mechanism
(d) A is  and is formed by SN2 displacement
and is formed by SN2 displacement
Ans. a
Sol. This reaction is supposed to be proceeds through addition elimination mechanism as shown below.

Correct answer is (a)
121. An optically active compound enriched with R-enantiomer (60% ee) exhibited  If the
If the  value of the sample is –135º, the ratio of R and S enantiomers would be
value of the sample is –135º, the ratio of R and S enantiomers would be
(a) R:S = 1:19
(b) R:S = 19:1
(c) R:S = 1:9
(d) R:S = 9:1
Ans. a
Sol. 60% enantiomeric excess of R-enantiomer gives +90º rotation.
Specific rotation of R enantiomer 
Hence, specific rotation of R-enantiomer = +150º
specific rotation of S-enantiomer = –150º.
Now, for sample optical rotation = –135º
Enantiomeric excess of S isomer 
Racemic part will be 10% (5% R-isomer, 5% S-isomer)
Total R = 5%, total S = 90 + 5 = 95%
Ratio R : S = 5 : 95 = 1 : 19
Correct answer is (a)
122. Match the amino acids with their structures:

(a) i-A, ii-E, iii-C
(b) i-C, ii-D, iii-B
(c) i-A, ii-B, iii-D
(d) i-C, ii-A, iii-B
Ans. d
Sol. Correct answer is (d)
123. Statement I: U(VI) is more stable than Nd(VI)
Statement II: The valence electrons in U are in 5f, 6d and 7s orbitals.
(a) Statement I and II are correct and Statement II is correct explanation of I.
(b) Statement I and II are correct but statement II is not an explanation for Statement I
(c) Statement I is correct and statement II is incorrect
(d) Statements I and II both are incorrect
Ans. b
Sol. Correct answer is (b)
124. The major products A and B in the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. Chemical reaction involved in the above transformation can be illustrated as


The last step in this reaction includes 1, 3 dipolar cycloaddition reaction also known as click reaction.
Correct answer is (d)
125. The major products A and B in the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (a)
126. An organic compound having molecular formula C15H14O exhibited the following 1H and 13C NMR spectral data.

(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. To solve this question we will calculate the DBE first


DBE = 9 indicates it may contain 2 benzene ring and one carbons group. Now lets look at NMR data

Two doublets in aromatic region indicates HOMO disubstituted benzene ring at ortho or hetero disubstituted benzene ring at para position.
Four 13C singlet in aromatic region confirms 4 types of aromatic carbon.
13C singlet at 190 indicates presence of 
Hence, compound is p-heterodisubstituted.

Correct answer is (d)
127. Identify appropriate reagents A and B in the following reactions

(a) A = LiAlH4, B = BH3·Me2S
(b) A = BH3·Me2S, B = LiAlH4
(c) A = LiBH4, B = BH3·Me2S
(d) A = BH3·Me2S, B = LiBH4
Ans. c
Sol. LiAlH4 reduces COOEt and COOH both group
BH3, Me2S reduced only COOH group
LiBH4 reduced only COOEt group.
Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (c)
128. The correct sequence of the reagent to be employed in the following transformation is

(a) (A) m – CPBA; (B) TsNHNH2; (C) AcOH; (D) H2, Pd / BaSO4
(b) (A) H2O2, NaOH (B) NH2NH2; (C) AcOH (D) H2, Pd / C
(c) (A) m – CPBA; (B) TsNHNH2; (C) NaOH; (D) H2, Pd / C
(d) (A) H2O2, NaOH; (B) TsNHNH2; (C) AcOH; (d) H2, Lindlar's catalyst
Ans. d
Sol. Chemical reaction involved in the above transformation can be illustrated as


Correct answer is (d)
129. Reaction of 11.6 g of the aldehyde A with 462 mg of Wilkinson's catalyst provided 9.2 g of alkene B. The mol % of the catalyst used and the yield of the reaction, approximately are

(a) 1.0 mol% and 80%
(b) 1.0 mol% and 90%
(c) 1.0 mol % and 90%
(d) 0.2 mol % and 80%
Ans. b
Sol. This problem can be solved using mole concept

The use of catalyst in term of percentage mole can be calculated as

Correct answer is (b)
130. The major products A and B in the following reaction sequene are
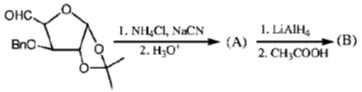
(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. Chemical reaction involved in the above transformation can be illustrated as

Above shown process is an example of strecker synthesis used in the preparation of amino acids.
Correct answer is (c)
131. The major products A and B in the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. d
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (d)
132. The major products A and B in the following raction sequence are

(a) 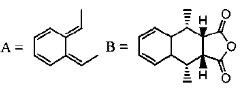
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (b)
133. Appropriate 1H NMR chemical shifts  for the protons A-D for the following compound are
for the protons A-D for the following compound are

(a) A–6.8; B–5.7; C–3.9; D–2.1 ppm
(b) A–6.8; B–5.7; C–2.1; D–3.9 ppm
(c) A–5.7, B–6.8; C–3.9; D–2.1 ppm
(d) A–5.7; B–6.8; C–2.1; D–3.9 ppm
Ans. d
Sol. Position of classical shift for different hydrogen in this case can be decided by using the concept of resonance as shown below.

Correct answer is (d)
134. The major product formed in the following reaction sequence is

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as


Correct answer is (b)
135. Citronellol A on oxidation with pyridinium chlorochromate (PCC) followed by treatment with aq. sodium hydroxide gives the product B (IR : 1680 cm–1); whereas oxidation with PCC in the presence of sodium acetate gives product C (IR: 1720 cm–1). Compound B and C are

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. PCC oxidise alcohol into carbonyl compound. Since the medium is acidic in PCC, so some side reaction may be takes place. To stop this side reaction normally buffer solution like sodium acetate is used.
Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (a)
136. Match the following starting compounds with corresponding products in photochemical reactions:

(a) i-E, ii-A, iii-B
(b) i-A, ii-C, iii-B
(c) i-D, ii-C, iii-A
(d) i-E, ii-A, iii-D
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (a)
137. The major product A and B in the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (a)
138. The major products A and B of the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (a)
139. The major products A and B in the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (b)
140. The major products A and B in the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (b)
141. The correct reagents for effecting the following reactions are

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as

Sulphonium ylide cause CH2 group transfer to carbonyl double bond while sulphoxonium group transfer CH2 group to conjugated double bond on the other hand CH2I2, Zn|Cu causes CH2 group transfer to isolated double bond commonly known as simmon's smith reaction.
Correct answer is (a)
142. The major product A and B of the following reaction sequence are

(a) 
(b) 
(c) 
(d) 
Ans. b
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (b)
143. The major product A and B in the following synthetic sequence are

(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (c)
144. The major products A and B in the following synthetic strategy are

(a) 
(b) 
(c) 
(d) 
Ans. a
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (a)
145. The product formed and the process involved in the following reactions are

(a) 
(b) 
(c) 
(d) 
Ans. c
Sol. Chemical reaction involved in the above transformation can be illustrated as

Correct answer is (c)
