The Complete Guide to Beta Decay, Gamma Decay, and Radioactive Decay
Nuclear chemistry is one of the most interesting and important subjects to study when you want to know more about the basic nature of matter. Nuclear reactions, on the other hand, change the nucleus, which is the very centre of the atom.
For science students, learning about Radioactive decay, Beta decay, and Gamma decays isn’t just about memorising definitions. It’s also about understanding how unstable elements change, how energy is released, and how we can use nuclear equations to predict these changes.
In this guide, we’ll look at the three main types of natural radioactivity, compare their properties, and learn how to balance nuclear reactions, which is an important skill.
What is the process of radioactive decay?
Radioactive decay is basically a process of breaking down. A lot of atomic nuclei are unstable on their own. These nuclei break down on their own by giving off particles in order to reach a more stable state. In these cases, the atom often changes its identity at its core; it actually becomes a different element.
This process is not the same as the chemical changes you might see in a test tube. Atoms keep their identity in chemical reactions because only electrons are shared or exchanged. In radioactive decay, though, the number of protons in the nucleus often changes, which changes the element’s identity.
Who is radioactive?
This instability doesn’t happen to every atom. But all nuclei with 84 or more protons are naturally radioactive. If they are unstable isotopes, elements with fewer than 84 protons can also be radioactive. These elements, whether Uranium-238 or Thorium-234, are always looking for stability through radioactive decay, beta decay, and gamma decay.
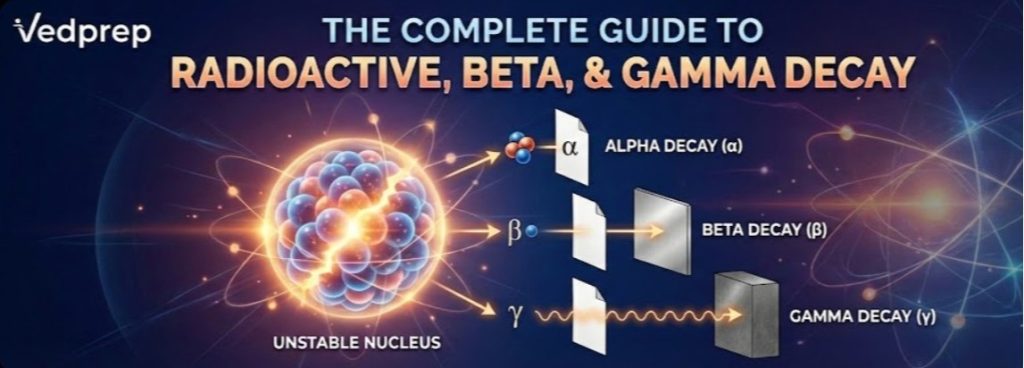
The “Big Three” Emissions are: Alpha, Beta, and Gamma
When natural radioactive decay happens, it usually sends out three different kinds of particles. In the past, scientists named them using the first three letters of the Greek alphabet before they knew what the particles were.
1. Alpha Decay: The Big One
In the world of radioactivity, the alpha particle is the “heavy lifter.”
Identity: An alpha particle is basically the nucleus of helium-4.
Composition: There are two protons and two neutrons in it.
The symbol for it is $\alpha$ or $\ce{_{2}^{4}He}$.
Charge: It has a +2 charge because it has two protons and no electrons.
Alpha emission is the most common type of radioactive decay in very heavy nuclei. The alpha particle is very heavy (about 8,000 times heavier than a beta particle), so when it is released, it makes the parent nucleus much less massive.
2. Beta Decay: The Nuclear Shapeshifter
Beta decay is a fascinating change in which the nucleus changes the ratio of neutrons to protons.
Identity: A beta particle is an electron with a lot of energy that comes straight from the nucleus.
The Paradox: You might wonder, “How can a nucleus give off an electron if it only has protons and neutrons?”
How It Works: A neutron in the nucleus breaks apart on its own during beta decay. This can be modelled as a neutron changing into a proton and an electron.
The outcome: The proton remains (raising the atomic number by one), and the electron is released as radiation.
It is shown as $\beta$ or $\ce{_{-1}^{0}e}$.
3. Gamma Decay: Only Energy
Alpha and beta decays involve particles, but gamma decays only involve electromagnetic radiation.
Identity: Gamma rays ($\gamma$) are very powerful photons that are similar to X-rays.
Mass and Charge: They have no mass and no charge.
Role: Gamma emission usually happens at the same time as other types of decay. When a nucleus gives off an alpha or beta particle, it is often left in a state of high energy and excitement. It gives off a gamma ray to “relax” to a lower energy level.
Prevalence: Almost all nuclear reactions talked about in academic texts give off gamma rays, even if they aren’t always written out in the equation for simplicity.
Comparative Analysis: Ionizing Power vs. Penetration Power
Students getting ready for tests like the CSIR NET need to know how radiation interacts with matter. We look at this by comparing two opposite things: Ionizing Power and Penetration Power.
The Principle of Trade-Off
In nuclear physics, a good rule of thumb is that the more mass and ionizing power something has, the less penetration power it has.
Power to Ionize: The Power to Hurt
Ionizing power is the ability of radiation to hit atoms and knock off electrons, which makes ions. This is what hurts DNA and living tissue.
Alpha particles have the most ionizing power because they are very heavy (4 amu) and have a charge. They are like “bulls in a china shop.” Upon contact, they hit molecules right away, causing a lot of damage in a very short distance.
Beta particles (intermediate): Because they are much smaller, they don’t interact as much as alpha particles, which means they don’t ionise as much.
Gamma Rays (Least Ionizing Power): They are the least ionizing because they don’t have mass or charge and don’t interact with most atoms.
Power of Penetration: The Ability to Get Through
This tells you how much material the radiation can go through before it stops.
Alpha Particles (Least Penetration): A sheet of paper, some clothes, or even the dead outer layer of human skin can stop them. Safety Note: Alpha particles are safe to touch but deadly if you breathe them in or eat them. Once they get inside the body, there is nothing to stop them from hurting delicate cells inside.
Beta Particles (Middle): These can go through paper, but wood or a thin sheet of aluminium (about 0.25 inches thick) usually stops them.
Gamma Rays (Most Penetrating): Gamma decay gives off rays that can go all the way through the human body. To effectively block them, you need several inches of thick lead.
Summary Table: Alpha, Beta, and Gamma Properties
| Particle | Symbol | Mass | Penetration Power | Ionizing Power | Shielding Required |
| Alpha | $\alpha$ | ~4 amu | Very Low | Very High | Paper / Skin |
| Beta | $\beta$ | ~0 amu | Intermediate | Intermediate | Aluminum / Wood |
| Gamma | $\gamma$ | 0 | Very High | Very Low | Lead |
Particle Symbol Mass Penetration Power Ionizing Power Shielding Needed
Alpha
Mastering Nuclear Accounting: How to Balance Equations
“Nuclear Accounting” is an important skill for any student who wants to learn about radioactive decay, beta decay, and gamma decay. Chemical equations must balance atoms, and nuclear equations must balance mass numbers and atomic numbers.
The Rules of Gold
Sum of Mass Numbers: The total mass number (top number, A) on the reactant side must be the same as the total on the product side.
Sum of Atomic Numbers: The total atomic number (bottom number, Z) on the reactant side must be the same as the total on the product side.
Don’t worry about Charge: In nuclear equations, we usually don’t worry about balancing electrical charge like we do in redox reactions. Instead, we only care about protons and neutrons.
Example 1: How to Balance Alpha Decay
Let’s take a look at how Uranium-238 ($\ce{^{238}U}$) decays.
Process: An alpha particle ($\ce{_{2}^{4}\alpha}$) comes from uranium-238.
The maths:
Change in mass number: $238 – 4 = 234$.
The atomic number changes from 92 (Uranium) to 90.
Find the product: Thorium (Th) is the element with atomic number 90.
Final Equation: $$\ce{_{92}^{238}U \rightarrow _{90}^{234}Th + _{2}^{4}\alpha}$$$$
Check: $234 + 4 = 238$ (mass matches) and $90 + 2 = 92$ (atomic number matches).
Example 2: Balancing the decay of beta particles
Think about Thorium-234 ($\ce{^{234}Th}$) going through beta decay.
How it works: A neutron changes into a proton and an electron ($\ce{_{-1}^{0}\beta}$).
The Numbers:
Mass Number Change: The electron has a mass number of 0, so the mass stays the same. $234$
The atomic number of an electron is -1. To make this balance, the product needs to have an atomic number that is one higher than the parent. $90 – (-1) = 91$.
Find the Product: Protactinium (Pa) is the element with atomic number 91.
The last equation is: $$\ce{_{90}^{234}Th \rightarrow _{91}^{234}Pa + _{-1}^{0}\beta}$$
Check: The mass matches because $234 + 0 = 234$ and the atomic number matches because $91 + (-1) = 90$.
Decay Series: The Way to Stability
A radioactive atom almost never becomes stable after just one emission. Radioactive decay, beta decay, and gamma decay usually happen in a series called a decay series.
The Path of Uranium-238
Uranium-238 doesn’t just give off one alpha particle and stop. It goes through fourteen different decay events over a long period of time before it becomes stable Lead-206.
Beginning: U-238 (Alpha decay) → Th-234
Next: Th-234, which is beta decay $\right arrow$ Pa-234
In the end, the series goes through Radium and Radon, and even makes Polonium, before ending up on Lead.
This idea explains why we still find things like Radon on Earth today. Any Radon that was on Earth when it was formed would have disappeared a long time ago because it has a short half-life. The decay of Uranium-238 in the soil keeps adding to the Radon we find now.
Why This Is Important: Health and Energy
The Scale of Energy
What makes nuclear reactions so much stronger than chemical ones? When chemical reactions happen, they break and rearrange bonds, which releases energy. This energy is usually in the range of kilojoules ($10^3$ kJ). Nuclear reactions let go of binding energy from the nucleus itself, and the amounts can be as high as $10^8$ kJ. A nuclear change takes almost a million times more energy per atom than a chemical change.
Effects on health
Radioactive decay, beta decay, and gamma decay all have different effects on living things depending on how much they are exposed to them.
Time and Distance: Limiting how long you are exposed and moving farther away from the source are the two best ways to protect yourself.
Inside vs. Outside: Keep in mind that gamma decays are dangerous even from a distance because they can get into your body, but alpha emitters are usually safe unless they get into your body through food, water, or air.
In short
To sum up the most important parts of radioactive decay, beta decay, and gamma decay:
Radioactivity is the process by which unstable nuclei break down on their own, which can change the element’s identity.
When alpha decay happens, a helium nucleus ($\ce{He}$) is released, which lowers the mass by 4 and the atomic number by 2. It can ionise things well, but it doesn’t go very deep.
Beta decay gives off a high-energy electron ($\ce{e}$), which doesn’t change the mass but does change the atomic number by 1. It has properties that are in between.
Gamma decay lets out high-energy photons ($\gamma$) that don’t have any mass or charge. It can get through things easily, but it doesn’t ionise very well.
The sum of the mass numbers and atomic numbers on both sides of a nuclear equation must be equal.
You can predict how nuclear materials will behave if you learn how to understand how radioactive decay, beta decay, and gamma decay work. This is a skill that is useful in many fields, including archaeology (carbon dating), medicine, and energy production.
Frequently asked Questions (FAQs)
What is radioactive decay?
Ans: Radioactive decay is the process where unstable atomic nuclei spontaneously decompose to reach a more stable state. In doing so, they emit particles and often transform into a different element.
Which elements are considered radioactive?
Ans: All nuclei with 84 or more protons are naturally radioactive. Elements with fewer than 84 protons can also be radioactive if they are unstable isotopes.
How is a nuclear reaction different from a chemical reaction?
Ans: In chemical reactions, atoms retain their identity because only electrons are shared or exchanged. In nuclear reactions, the nucleus itself changes (often the number of protons), which changes the identity of the element.
Why do nuclei undergo radioactive decay?
Ans: Nuclei are unstable and undergo decay to achieve a stable state. If a single decay doesn't achieve stability, a series of decays will occur until a stable nucleus is formed.
What is an alpha particle made of?
Ans: An alpha particle is essentially a helium-4 nucleus, consisting of two protons and two neutrons.
How does alpha decay change the nucleus?
Ans: When a nucleus emits an alpha particle, its mass number decreases by 4 and its atomic number decreases by 2.
What is a beta particle?
Ans: A beta particle is a high-energy electron that is ejected directly from the nucleus.
How can a nucleus emit an electron if it doesn't contain any?
Ans: During beta decay, a neutron inside the nucleus splits into a proton and an electron. The proton stays in the nucleus, while the electron is ejected.
How does beta decay affect the atomic number?
Ans: Since a neutron turns into a proton, the atomic number of the atom increases by one, while the mass number remains the same.
What are gamma rays?
Ans: Gamma rays are high-energy electromagnetic radiation (photons) with no mass and no charge. They are similar to X-rays but much more powerful.
When does gamma decay typically occur?
Ans: Gamma emission usually accompanies alpha or beta decay. It happens when a nucleus is left in an excited, high-energy state and emits energy to "relax" to a lower state.
Which type of radiation has the highest ionizing power?
Ans: Alpha particles have the highest ionizing power because of their large mass and +2 charge, allowing them to damage tissue significantly upon contact.
Which type of radiation is the most penetrating?
Ans: Gamma rays are the most penetrating. They can pass completely through the human body and require dense materials like lead to block them.
How can you shield yourself from beta particles?
Ans: Beta particles can penetrate paper but are typically blocked by wood or a thin sheet of aluminum (about 0.25 inches thick)
If alpha particles are easily blocked, why are they dangerous?
Ans: Alpha particles are stopped by skin, so they are safe externally. However, if inhaled or ingested, they are extremely dangerous because they have no barrier to stop them from damaging sensitive internal cells.