The Photoelectric Effect in 2026: From Einstein’s Nobel Prize to the Heart of Modern Quantum Technology
We are standing on the shoulders of giants in 2026. The quantum revolution is no longer just a theory; it’s what makes our lives work every day. The Photoelectric Effect is what makes everything possible, from the super-efficient solar panels on our roofs to the night-vision sensors in our self-driving cars.
Albert Einstein won the Nobel Prize for explaining this phenomenon over a hundred years ago, but it has become much more important in the 2020s. Students getting ready for tests like the CSIR NET, GATE, or IIT JAM are not just learning about an experiment from the past; they are also learning how to work in the 21st century.
But most textbooks still teach the Photoelectric Effect as a long-forgotten part of history. They talk about plates of zinc and leaves of gold. We will break that mold in this long guide. We will explore the Photoelectric Effect through the lens of 2026, discussing “Attosecond Physics,” advanced photovoltaics, and the deep quantum mechanics that your competitors’ blogs miss.
Why We Needed a Revolution: The Failure of Classical Wave Theory
You need to know why the Photoelectric Effect confused the smartest people of the 1800s before you can understand it. Classical physics believed light was a wave, like a ripple in a pond. If this were true, the Photoelectric Effect should have obeyed certain laws. It broke every single one.
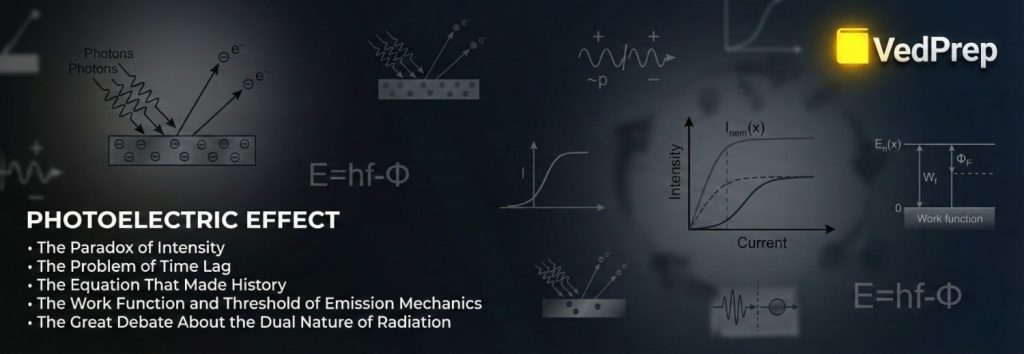
The Paradox of Intensity
The classical prediction says that if light is a wave, making it brighter also makes it more powerful. So, any very bright light, no matter what color it is, should eventually knock out electrons.
The Truth: You can shine a very bright red light on a metal surface for years and nothing will happen. But a dim, weak blue light will instantly push out electrons. The Photoelectric Effect depends on frequency, not intensity.
The Problem of Time Lag
Classical Prediction: A wave’s energy needs to “build up.” It should take time for an electron to soak up enough energy to escape.
The Truth: The emission happens right away (in less than $10^{-9}$ seconds). There is no “soaking up.” It’s a hit or miss.
The photon, the basic particle of light, was born out of this crisis.
Einstein’s Quantum Leap: Light as Particles
Einstein put forth a revolutionary concept in 1905: Light is not a continuous wave cascading over the beach; it is a barrage of bullets. A quantum or photon is a packet of energy that makes up each “bullet.”
The Equation That Made History
The energy of these photons is quantized:$E = h\nu$
$E$ is energy h$ is Planck’s constant, which is 6.626 \times 10^{-34}$ Js. Frequency is ν (nu).
This simple equation made the Photoelectric Effect possible. An electron is only released when a single photon strikes it with sufficient energy to sever its bond. It’s a 1-on-1 collision. If the photon has a low frequency (like red light), it’s like throwing a ping-pong ball at a brick wall: nothing happens. If it has a high frequency (UV light), it’s like a cannonball hitting the wall.
The Work Function and Threshold of Emission Mechanics
For the 2026 exams, it’s very important to know the jargon. The Photoelectric Effect is determined by the characteristics of the material.
Function of Work ($\Phi$)
This is the “Exit Fee.” Each metal holds onto its electrons with a different amount of strength. The electron has to pay this energy cost to get away.
Cesium: Low work function ($2.14$ eV). Simple to ionize. Used for seeing at night.
Platinum has a high work function of $5.65$ eV. Hard to ionize.
Einstein’s Photoelectric Equation $K_{max} = h\nu – \Phi$ $K_{max}$: The maximum kinetic energy of the electron that was ejected.hν: The energy that the photon brings (Income).Φ: The energy that is paid to get out (Tax).
The Photoelectric Effect doesn’t happen if $h\nu < \Phi$. Time.
Experimental Study: The Lenard Configuration
Philipp Lenard proved Einstein’s theory. Even in university labs in 2026, his experimental setup is the standard for testing the Photoelectric Effect.
Potential to Stop ($V_0$)
This is the most important idea for math problems.
Electrons fly across the tube when you eject them. You push them away if you give the collector plate a negative potential. The Stopping Potential is the voltage needed to stop even the fastest electron.$eV_0 = K_{max} = h\nu – \Phi$
Important point: The stopping potential only depends on the frequency of light, not its intensity. More light makes more electrons (current), but it doesn’t make them move faster.
The 2026 Summary of the Laws of Photoelectric Emission
Here are the four unchangeable laws of the Photoelectric Effect for a quick review:
Instantaneous Process: There is no delay.
Intensity $\propto$ Right now: Twice as bright → Twice as many photons → Saturation Current: The number of electrons ejected is twice as many.
The frequency is proportional to the energy: to raise the “color” frequency, make electrons move faster (Kinetic Energy).
Threshold Frequency ($\nu_0$): No emission happens below this frequency, no matter how strong it is.
The Great Debate About the Dual Nature of Radiation
The Photoelectric Effect showed that light is a particle. But diffraction shows that light is a wave.
We agree with the “Dual Nature” in 2026. Light is a “wavicle.”
Light travels in waves, which can cause interference and diffraction.
Interaction: Light hits things like a particle (Photoelectric Effect, Compton Effect).
This duality is what makes Quantum Mechanics work.
Applications in 2026: More Than Just the Textbook
You need to update your knowledge here. It’s not just photocells that are affected by the photoelectric effect anymore.
Perovskites are advanced photovoltaics.
Perovskites will take the place of silicon solar panels in 2026. These materials have been adjusted to use the Photoelectric Effect more effectively by capturing a wider range of light. Quantum tuning of the work function has increased efficiency from 22% to more than 35%.
Lidar and Night Vision
In 2026, self-driving cars will use Lidar (Light Detection and Ranging). The sensors use the Photoelectric Effect to instantly detect laser photons that have bounced off of something, which lets the car “see” a person in complete darkness.
Photoelectron Spectroscopy (PES)
This is the “fingerprint scanner” for molecules. Scientists can figure out the electronic structure of new drugs and superconductors by firing X-rays at a material and measuring the kinetic energy of the electrons that come out. In 2026, it will be the most important tool for material science.
Physics at the Attosecond Level
This is the most advanced. In 2026, scientists use the Photoelectric Effect to measure events that happen in attoseconds ($10^{-18}$ seconds). We can see electrons move around inside an atom in real time.
A numerical strategy for tests that are competitive
If you want to pass the CSIR NET or GATE, you need to know more than just the theory. You have to figure things out. These are the most common mistakes people make when answering Photoelectric Effect questions.
Unit Conversion Trap: The question gives Wavelength ($\lambda$) in Angstroms ($Å$) and Work Function ($\Phi$) in Electron-Volts (eV).
To find the answer, use the shortcut formula: $$E (in\ eV) = \frac{12400}{\lambda\ (in\ Å)}$$
Unless someone asks you to, don’t waste time changing everything to Joules
The Questions About Graphs
Graphs are great for tests.
Graph: Stopping Potential ($V_0$) vs. Frequency ($\nu$).
The slope of this line is always $h/e$, which is Planck’s constant divided by the charge of an electron. It is a constant that is true everywhere.
The Y-intercept is -\Phi/e. This tells you how much work the metal can do.
VedPrep: Learning Quantum Mechanics
The Photoelectric Effect is the first step into Quantum Mechanics. It’s easy to understand but hard to do the math. If you make a mistake with the signs in the stopping potential calculation, you lose points.
At this point, VedPrep becomes your business partner.
We teach physics for the year 2026 at VedPrep.
Visualization Modules: Don’t just think about electrons jumping; see them. You can change the frequency and intensity of our interactive simulations to see how the stopping potential changes in real time.
Shortcuts for solving problems: We show you the “Unit Hacks,” such as the 12400 rule, that can help you save time on tests like GATE and JAM.
Experimental Insight: Our modules cover the modern experimental details that are often asked in high-level interviews, such as how surface impurities affect Work Function.
Integration of Current Events: We connect the Photoelectric Effect to the most recent Nobel Prizes, such as the 2023 Nobel Prize in Physics for Attosecond pulses, so that your answers are up-to-date and impressive.
You don’t just memorize formulas with VedPrep; you really understand the physics. Let us help you make this basic subject your best weapon.
Conclusion
The Photoelectric Effect is what made it possible for us to touch the quantum world. It showed that energy comes in packets, that nature is made up of small parts, and that the rules that work for big things don’t work for small things.
We are still using the key that Einstein gave us in 1905, even though we are building quantum computers and using the sun in ways that have never been done before. For a science student, understanding the Photoelectric Effect is a must. It is the modern physics alphabet.
When a sensor opens a door for you or a solar panel powers your home, think about how the photon and electron dance. Keep in mind the photoelectric effect.
Frequently Asked Questions (FAQs)
Why did the Photoelectric Effect prove Classical Wave Theory wrong?
Ans: Classical physics viewed light as a continuous wave, predicting that brighter light (higher intensity) would eventually eject electrons regardless of color, and that energy would need time to "build up". The Photoelectric Effect disproved this by showing that emission depends on frequency (color), not intensity, and happens instantly.
What is the "Paradox of Intensity"?
Ans: The paradox is the contradiction between classical prediction and experimental reality. Classical theory predicted that very bright light should knock out electrons because it has more power. However, the reality is that a bright red light (low frequency) will never eject electrons, while a dim blue light (high frequency) will do so instantly.
What is the "Problem of Time Lag" mentioned in the text?
Ans: Classical theory suggested electrons needed time to "soak up" enough energy from a wave to escape. In reality, the Photoelectric Effect is instantaneous, occurring in less than $10^{-9}$ seconds, proving light acts like a "bullet" (photon) rather than a continuous wave.
How does Einstein’s view of light differ from the classical view?
Ans: Einstein proposed in 1905 that light is not a continuous wave but a "barrage of bullets" called quanta or photons. He established that the energy of these photons is quantized and calculated as $E = h\nu$.
What is the Work Function ($\Phi$)?
Ans: The Work Function is described as the "Exit Fee" or the specific energy cost an electron must pay to escape a particular metal. Different metals have different values; for example, Cesium has a low work function ($2.14$ eV), while Platinum has a high one ($5.65$ eV).
What is the mathematical formula for Einstein's Photoelectric Equation?
Ans: The equation is $K_{max} = h\nu - \Phi$. Here, $K_{max}$ is the maximum kinetic energy of the ejected electron, $h\nu$ is the incoming photon's energy (Income), and $\Phi$ is the Work Function (Tax) paid to leave the metal.
What is Stopping Potential ($V_0$)?
Ans: Stopping Potential is the negative voltage applied to a collector plate required to stop even the fastest ejected electron from reaching it. It is a critical concept for math problems and depends solely on the frequency of the light, not its intensity.
Does increasing light intensity affect the Stopping Potential?
Ans: No. Increasing the intensity (brightness) only increases the number of photons, which leads to more electrons being ejected (higher saturation current). It does not make the electrons move faster or change the stopping potential
What is the "Threshold Frequency"?
Ans: The Threshold Frequency ($\nu_0$) is the minimum frequency required to eject an electron. Below this specific frequency, no emission will occur, regardless of how intense or bright the light source is.
How is the Photoelectric Effect used in solar energy in 2026?
Ans: It is used in advanced photovoltaics made of Perovskites, which are replacing silicon panels. By using "Quantum tuning" of the work function, these materials have increased solar panel efficiency from 22% to over 35%.