Stabilizing Interactions: What They Are, What Kinds There Are, and How They Work in Biological Systems
Meta Description: Learn about the world of stabilizing interactions. Learn about the different types of bonds and forces that keep biomolecules working, from covalent bonds to Van der Waals forces.
In the big and complicated world of biology, structure is often what makes the difference between a living cell that works and a messy mix of chemicals. But what keeps these structures from falling apart? The answer is based on a basic idea called the stabilizing interaction. These invisible forces work like glue to keep biomolecules like proteins, DNA, and lipids in their proper shapes and carry out the functions that are necessary for life.
It’s important to know the types, definitions, and uses of these forces whether you’re a student getting ready for a tough test or a researcher who wants to review the basics. We’ll go into great detail about the physics and biology of these interactions in this complete guide. We’ll look at everything from the strong covalent backbones to the tiny changes in Van der Waals forces.
What does it mean to have a stabilizing interactions?
A stabilizing interaction is the total of all the weak and strong bonds that form between atoms and molecules. These interactions keep molecules in balance and build the complex structures that make up living things.
In simple terms, stabilizing interactions helps molecules pull or push each other in a way that keeps them folded and working right. Life’s delicate machinery would break down without these forces. For example, changing or getting rid of the stabilizing interactions that keep molecules in a certain shape often causes denaturation. This loss of structure is what causes many life processes, like metabolism and replication, to stop working.
The Definition and Main Idea of stabilizing interactions
The stabilizing interactions is the molecular interaction that is necessary for keeping and controlling the specific structure of big biological molecules like proteins, lipids, and nucleic acids. The stability of these molecules is what makes them work in the body.
Scientists have learned more about important biological processes by studying these interactions, such as:
How a long chain of amino acids folds into a working 3D shape is called protein folding.
What keeps the famous double helix of DNA together?
Enzymatic Reactions: The process by which enzymes attach to substrates to speed up reactions.
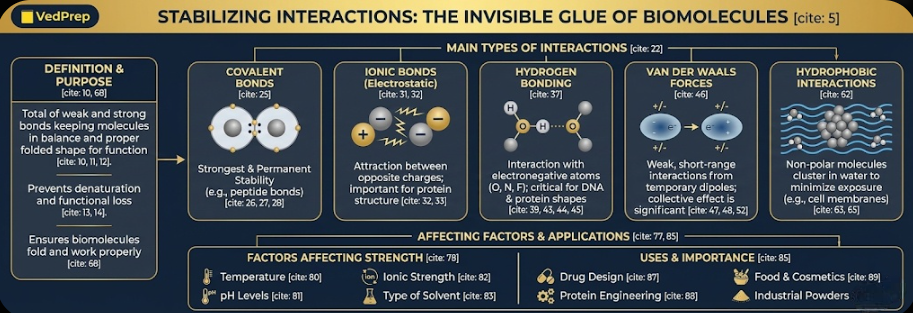
The Main Types of Interactions That Keep Things Stable
In order to comprehend the stability of biomolecules, it is essential to classify the various types of stabilizing interactions. These have different strengths and functions, but they all work together to keep cells in balance.
Covalent Bonds: The Permanent Structure of stabilizing interactions
When atoms share pairs of electrons, they form covalent bonds, which are the strongest type of chemical bond in biomolecules. Covalent bonds give the molecular backbone permanent stability when they are part of a stabilizing interaction.
For example, the phosphodiester bond in nucleic acids and the peptide bond in proteins.
Role: Covalent bonds are the strongest and most stabilizing interactions. They are the building blocks that hold all other stabilizing forces together.
Ionic Bonds (Electrostatic Interactions)
Electrostatic interactions, also known as ionic bonds, happen between ions with opposite charges. They are not as strong as covalent bonds, but they are very important for keeping proteins’ tertiary and quaternary structures stable.
These bonds happen in proteins between the side chains of amino acids that have a positive charge and a negative charge. This kind of stabilizing interaction helps molecules stay in their right folded state by making them pull on or push away from each other. In scientific terms, electrostatic attraction is when ions interact with each other or with things that aren’t charged.
Bonding with Hydrogen
Hydrogen bonding is one of the most well-known ways that molecules in biology can stay stable. When a hydrogen atom is attached to an electronegative atom, like oxygen or nitrogen, and interacts with another electronegative atom nearby, this happens.
Strength: Hydrogen bonds are not as strong as covalent bonds, but they are strong enough to hold important structures together. A hydrogen bond can be as strong as 4 kJ/mol or as weak as 50 kJ/mol.
Importance of stabilizing interactions:
In proteins, they keep alpha-helices and beta-sheets together.
In DNA, they link the two strands by pairing bases: adenine with thymine and guanine with cytosine.
Only certain atoms, like oxygen (O), fluorine (F), and nitrogen (N), can make these bonds with hydrogen atoms.
Forces of Van der Waals
Van der Waals forces are weak, short-range stabilizing interactions that happen when dipoles are temporarily formed. They are the weakest interactions, but when they all work together, they have a big effect.
The Physics of Van der Waals
In physical chemistry, the Van der Waals force is the total of all the forces that pull molecules together or push them apart that aren’t caused by hydrogen or covalent bonds. These forces come from electrical interactions that happen between two particles. When negatively charged electrons in one atom get closer to another atom, the electron clouds around them change shape in real time, making them temporarily attracted to each other.
There are different kinds of forces in the Van der Waals group:
Dipole-Dipole Interactions: These happen between polar molecules that have dipoles that don’t change. They happen when the negative part of one molecule interacts with the positive part of another molecule.
London Forces of Dispersion: These are the weakest types of bonds, and they happen when short-term dipoles are created. They make non-polar substances turn into liquids.
Keesom Force: A major supporter of Waals communications that involve dipoles, multipoles, or quadrupoles repelling or attracting each other.
Debye Force: This is what causes molecules to attract each other when they have a polarity that has been started and will last.
The Van der Waals equation helps us figure out how real gases behave in these ways:
$$nRT=(P+n^2a/V^2)(V-nb)$$
In this equation, $b$ is the volume of gases that are not taken into account, and the strength of attraction between them is also taken into account.
Interactions that don’t like water
Hydrophobic interactions are the way that non-polar molecules come together in water to avoid being in it as much as possible. In water, hydrophobic groups stick together inside the molecule, while hydrophilic groups stick together with the water on the outside.
This stabilizing interactions is what makes cell membranes possible. Lipids line up to make a bilayer.
Biological Importance: The Intersection of Structure and Function
The main biological purpose of any stabilizing interaction is to make sure that biomolecules fold and work properly. Cells would fall apart and genetic material would become unstable if these forces weren’t there.
The Structure and Stability of Proteins
These interactions are very important for proteins at every level of their structure:
Primary Structure: Connected by peptide bonds that are covalent.
Secondary Structure: Hydrogen Bonding keeps it stable (alpha-helices and beta-sheets).
Tertiary Structure: The final folded shape is held together by a complicated mix of hydrophobic interactions, Van der Waals forces, and electrostatic bonds.
Quaternary Structure: Hydrophobic and ionic interactions hold together many protein chains.
Genetic Engineering and DNA
The famous double-helix shape of DNA is held together by hydrogen bonds between bases and Van der Waals forces between stacked bases. This stability is very important for genetic engineering, where these forces are used to stabilize DNA and stop it from breaking down.
Things that affect stabilizing interactions
The strength of a stabilizing interaction can be affected by many things outside of the interaction itself. To keep biological stability in the lab and in the clinic, it’s important to know these things.
Temperature: High temperatures can break weak bonds, like hydrogen bonds and Van der Waals forces, which can cause proteins to lose their shape.
pH Levels: Changes in pH can change the way amino acids interact with each other by changing the charges on them.
Ionic Strength: Too many ions in a solution can change the electrostatic forces of attraction, which can break up stabilizing interactions.
Type of Solvent: Water is the best solvent for hydrogen bonds and interactions that are not water-soluble. Ethanol and other organic solvents tend to break up these interactions.
Uses of Stabilizing Interactions
The kinds, meanings, and uses of these forces go far beyond what you can find in basic biology books. Many industrial and technological processes use them.
Drug Design: Scientists can make drugs that bind to specific proteins to stop them from working by understanding how molecules interact with each other.
Protein Engineering: Scientists change the way proteins are structured by controlling how they interact with each other, such as through hydrogen bonding and hydrophobic forces.
Food and Cosmetics: Interactions that stabilize help keep the quality and texture of food and beauty products that are made from living things.
Industrial Powders: It’s interesting that Van der Waals forces are stronger than gravity for groups of very small particles, like very fine-grained dry powders.
Is CSIR NET Hard? A Vedprep Point of View
A lot of people who want to take the CSIR NET ask, “Is it hard?” The answer is often in how well you understand the concept. Stabilizing Interactions and other topics like them are very important to the Life Sciences curriculum. The test doesn’t just check how well you remember things; it also checks how well you can use these ideas to solve hard biological problems.
At Vedprep, we think that learning the basic “types of definition and application” of biomolecular forces makes a tough subject easier to understand. Our specialized courses break down these complicated physics-based biological ideas into simple modules so that you don’t just memorize facts but also understand the “invisible glue” that keeps life and your exam preparation together.
Frequently Asked Questions (FAQs)
What is a stabilizing interaction?
Ans: It is the sum of all weak and strong bonds forming between atoms and molecules that keep them balanced and build complex structures in living things .
Why are stabilizing interactions important for life?
Ans: These invisible forces act like glue to keep biomolecules like proteins, DNA, and lipids in their proper shapes so they can function
What happens when stabilizing interactions are disrupted?
Ans: Removing or changing these interactions often causes denaturation (loss of structure), which stops life processes like metabolism and replication .
What are the three main examples of biological processes relying on these interactions?
Ans: They include protein folding, holding the DNA double helix together, and enzymatic reactions where enzymes attach to substrates .
Which is the strongest type of stabilizing interaction?
Ans: Covalent bonds are the strongest type, providing permanent stability to the molecular backbone .
What are ionic bonds (electrostatic interactions)?
Ans: These are interactions that happen between ions with opposite charges, such as between positive and negative side chains of amino acids in proteins.
How do ionic bonds compare to covalent bonds?
Ans: Ionic bonds are not as strong as covalent bonds but are vital for stabilizing the tertiary and quaternary structures of proteins.
What is a hydrogen bond?
Ans: It occurs when a hydrogen atom attached to an electronegative atom (like oxygen or nitrogen) interacts with another nearby electronegative atom
How strong is a hydrogen bond?
Ans: A hydrogen bond is weaker than a covalent bond, ranging from roughly $4~kJ/mol$ to $50~kJ/mol$ 10.
What is the role of hydrogen bonds in DNA?
Ans: They link the two strands of DNA by pairing bases: adenine with thymine and guanine with cytosine.
Which atoms can form hydrogen bonds?
Ans: Only certain electronegative atoms like oxygen (O), fluorine (F), and nitrogen (N) can form these bonds with hydrogen.
What are Van der Waals forces?
Ans: They are weak, short-range interactions caused by temporary or permanent dipoles formed between particles.
What are the different types of Van der Waals forces mentioned?
Ans: The types include Dipole-Dipole interactions, London Dispersion forces, Keesom force, and Debye force.
What are hydrophobic interactions?
Ans: These occur when non-polar molecules aggregate in water to avoid contact with it, such as hydrophobic groups sticking together inside a molecule .
How do hydrophobic interactions contribute to cell structure?
Ans: They are responsible for making cell membranes possible by causing lipids to line up into a bilayer.