What are Electron Configurations?
The Electron or Electronic Configuration of an element describes the arrangements and distribution of electrons in its atomic orbits.
The configuration of electrons for elements follows certain rules and standard notation. Let\’s see how the sequence of the configuration follows certain rules.
The (n + l) rule
The orbital with a subshell having a lower (n + ℓ) value will be filled first.
Case I: (n1 + ℓ1) < (n2 + ℓ2)
1s 2s
(n + ℓ) 1 2
∴ 1s will be filled prior to 2s.
Case II: (n1 + ℓ1) = (n2 + ℓ2) but n1 < n2
Then lower the value of the principal quantum number, the lower will the energy of the subshell, and will be filled prior.
3d 4p
(n + ℓ) 5 5
but (n = 3) < (n = 4), so 3d will be filled prior to 4p.
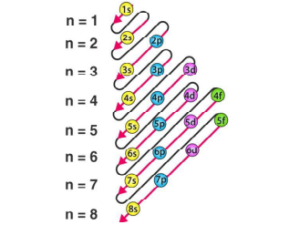
Aufbau Principle
It states that the electron is placed in order to give the lowest total energy to the atom. In ordinary words, electrons are added progressively to the various orbitals in their order of increasing energy starting with the orbital of lowest energy. The energy of orbitals can be predicted using the (n + ℓ) rule.
The order of increasing energy may be summed up as follows—
1s, 2s, 2p, 3s, 4s,3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d …..
Pauli’s Exclusion Principle
It states “No two electrons in the same atom may have the same set of n, ℓ, m, and s quantum numbers”. It follows that each orbital can accommodate a maximum of two electrons with different values of spin quantum number.
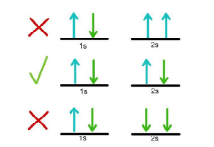
Example: Consider an electronic arrangement in 1st energy level (n = 1). For n = 1, ℓ= 0, and m = 0. Now s can have two values corresponding to each value of m i.e. s = +1/2, –1/2 (n, 1, possible designation of an electron in a state with n = 1 is 1, 0, 0, +1/2 and 1, 0, 0, –1/2 (n, 1, m, s) i.e., two quantized states.
Hund’s Rule of Maximum Multiplicity
According to Hund’s rule, the electrons are placed in orbitals so as to give a maximum total spin value (or a maximum number of parallel spins). This rule is a consequence of the energy required for pairing electrons in the same orbital.
This means an electron always occupied a vacant orbital in the same sub-shell (degenerate orbital) and pairing starts only when all of the degenerate orbitals are filled up.
This means that the pairing starts with the 2nd electron in the s sub-shell, the 4th electron in the p-subshell, the 6th electron in d-subshell, and the 8th electron in the f sub-shell.
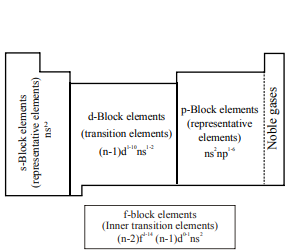
Electron Configuration in the Long Form Periodic Table
The periodic table is divided into four main blocks. These are s, p, d and f-blocks. The classification of elements into blocks is primarily based upon their electronic configuration.